USDA confirmed highly pathogenic H7N3 avian influenza in a commercial flock in Chesterfield County, South Carolina
On Apr. 9, 2020, the U.S. Department of Agriculture (USDA) confirmed the presence of highly pathogenic H7N3 avian…

On Apr. 9, 2020, the U.S. Department of Agriculture (USDA) confirmed the presence of highly pathogenic H7N3 avian…
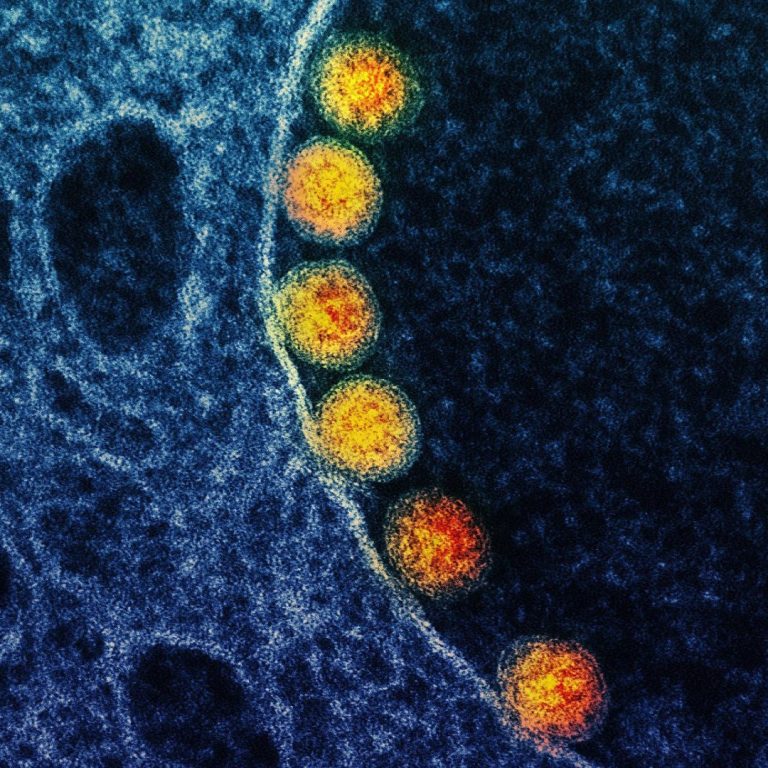
On Apr. 4, 2020, Mesa Biotech announced the addition of the novel coronavirus (SARS-CoV-2) to its active influenza…

On Mar. 20, 2020, a study was published in JAMA that expands the understanding of influenza-associated complications. Influenza…
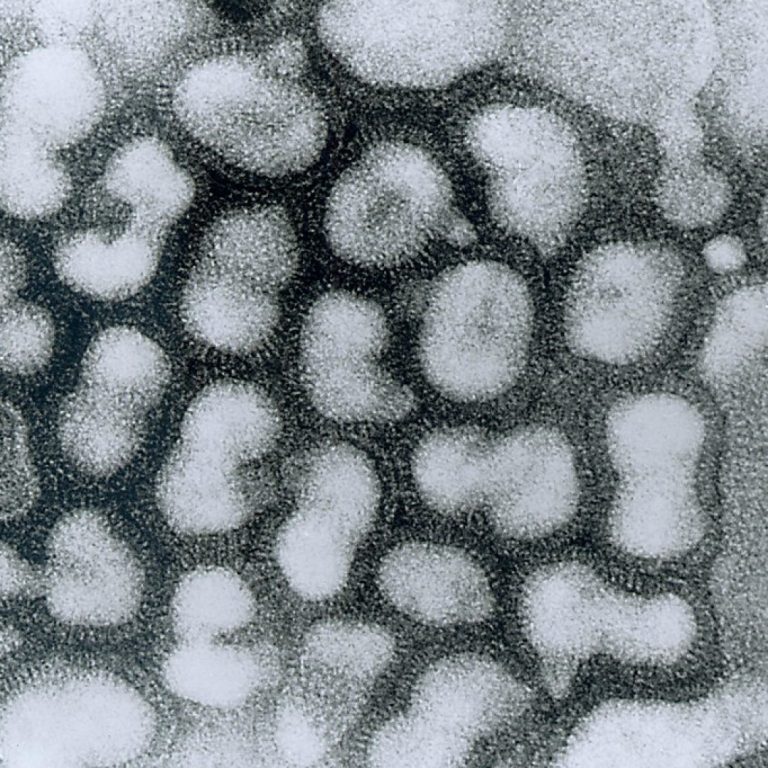
On Dec. 12, 2019, BioAegis Therapeutics announced publication of new research findings with recombinant human plasma gelsolin in…
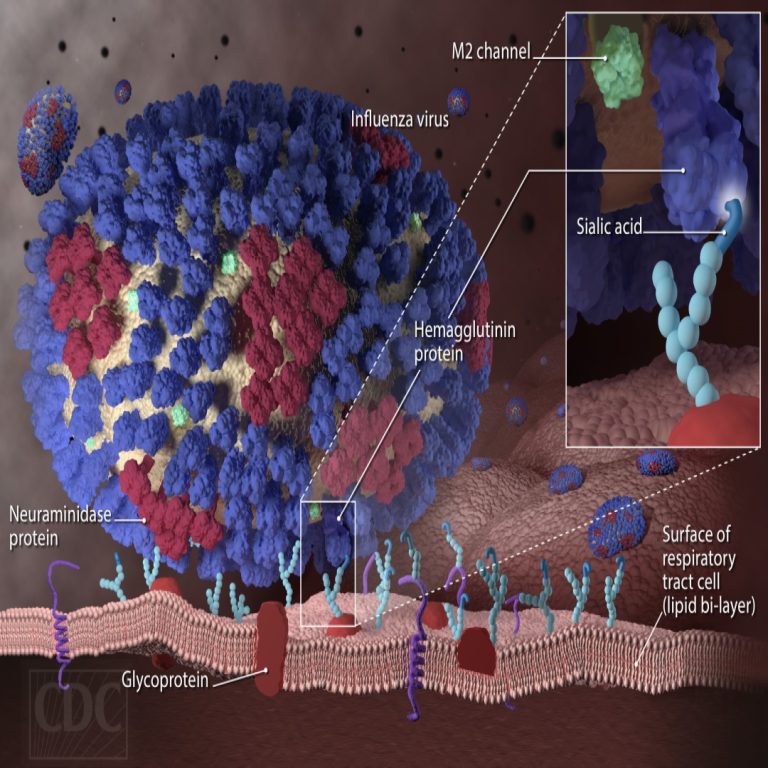
On Sept. 30, 2019, the University of Georgia (UGA) announced it had signed a contract with the National…
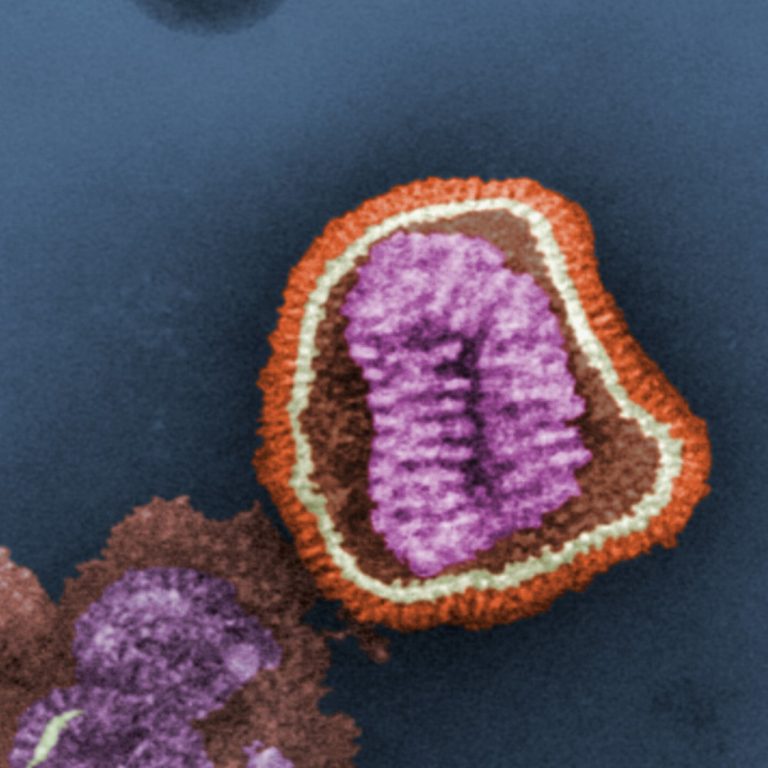
In Jun. 2019, Defense Advanced Research Projects Agency (DARPA) announced it had funded a $21.9 gene modulation research…

On Jun. 5, 2019, Vaccitech announced that it has administered its pandemic universal influenza A vaccine MVA-NP+M1 (VTP-100)…
On Jan. 23, 2019, U.S. Food and Drug Administration (FDA) announced it had approved approved the use of…

On Aug. 24, 2018, the Centers for Disease Control and Prevention (CDC) published Immunization Practices Advisory Committee’s (ACIP)…
On Jun. 8, 2018, the U.S. Centers for Disease Control and Prevention (CDC) published Immunization Practices Advisory Committee…

On Apr. 23, 2018, Medigen Vaccine Biologics and GC Pharma entered an exclusive distribution agreement for GCC’s quadrivalent…

On Apr. 1, 2018, the American College of Obstetricians and Gynecologists released a committee opinion on influenza vaccination…
On Jan. 11, 2018, the U.S. Food and Drug Administration (FDA) approved an expanded indication for the four-strain…

On Aug. 25, 2017, the U.S. Centers for Disease Control and Prevention (CDC) published Immunization Practices Advisory Committee…
On Feb. 28, 2014, the U.S. Centers for Disease Control and Prevention (CDC) published Immunization Practices Advisory Committee…

On Dec. 14, 2012, the U.S. Food and Drug Administration (FDA) announced it had approved GlaxoSmithKline’s quadrivalent formulation…

On Nov. 20, 2012, the FDA approved a seasonal flu vaccine produced by Novartis using animal cell culture…
On Nov. 20, 2012, the U.S. Food and Drug Administration (FDA) announced it had approved first seasonal influenza…

On Feb. 21, 2012, as part of a national collaboration, Oregon Health & Science University researchers were studying…

On Aug. 10, 2010, WHO Director-General Dr Margaret Chan announced that the H1N1 influenza virus had moved into…

On Feb. 24, 2010, the Immunization Practices Advisory Committee (ACIP) recommended universal Influenza vaccination for those 6 months…
On Dec. 23, 2009, the FDA approved high-dose inactivated influenza vaccine (Fluzone High-Dose) for people ages 65 years…

On Oct. 24, 2009, President Barack Obama declared the swine flu (N1n1) outbreak a national emergency. The declaration…

On Jul. 31, 2009, the Immunization Practices Advisory Committee (ACIP) updates the 2008 recommendations regarding the use of…
On Jun. 11, 2009, the Dr. Margaret Chan, Director-General World Health Organization (WHO), declared the world was at…

On May 22, 2009, the U.S. Dept. of Health and Human Services (HHS) directed $1 billion toward development…

On Apr. 15, 2009, a novel influenza A virus (then referred to as ‘swine origin influenza A virus’)…

On Apr. 15, 2009, infection with the new influenza A virus (then referred to as ‘swine origin influenza…

On Apr. 15, 2009, the U.S. Centers for Disease Control and Prevention (CDC) identified the novel H1N1 influenza…

In Apr. 2009, physicians used point of care rapid immunoassay tests to provide influenza results within 15 minutes…