A combined hepatitis A inactivated and hepatitis B (recombinant) vaccine was licensed
On May 11, 2001, the Food and Drug Administration (FDA) licensed a combined hepatitis A and B vaccine…
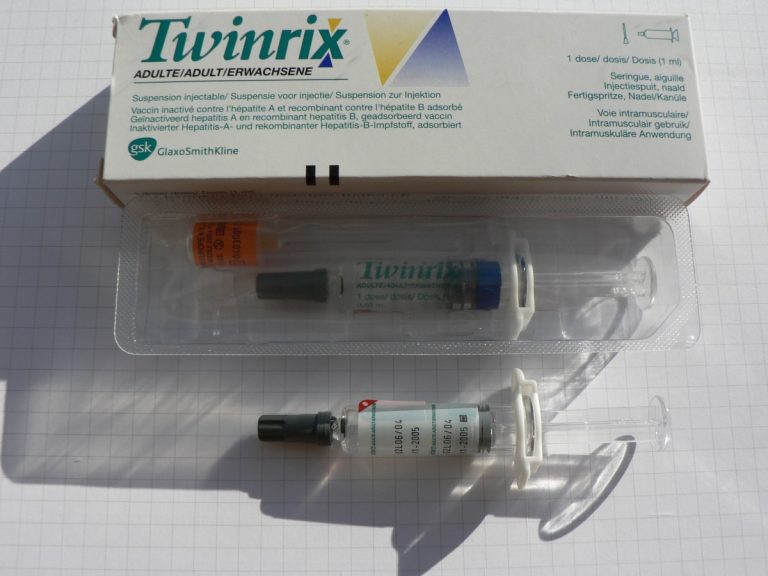
On May 11, 2001, the Food and Drug Administration (FDA) licensed a combined hepatitis A and B vaccine…

On Aug. 29, 1999, Merck, West Point, Pennsylvania) received approval from the FDA of a supplement to Merck’s…

On Jan. 15. 1999, the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices…

On Aug. 26, 1998, Bill and Melinda Gates announced the establishment of the Bill and Melinda Gates Children’s Vaccine…
In 1998, some research caused concern that hepatitis B vaccination might be linked with multiple sclerosis (MS), a…

On Oct. 2, 1996, the Food and Drug Administration (FDA) licensed a combined Hib conjugate and hepatitis B…

On Mar. 29, 1996, the second inactivated hepatitis A vaccine (Vaqta by Merck) was licensed.

On Feb. 22, 1995, the first inactivated hepatitis A vaccine, distributed by SmithKline Beecham Pharmaceuticals (Philadelphia, Pennsylvania), was…

On Nov. 22, 1991, the Immunization Practices Advisory Committee (ACIP) published recommendations for routine hepatitis B vaccination for…
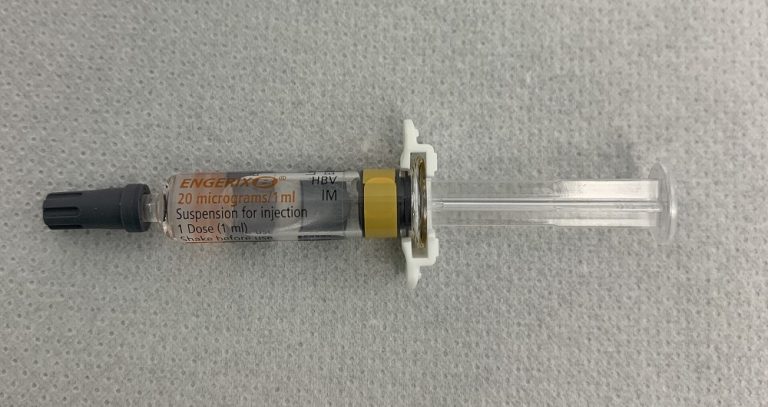
On Aug. 28, 1989, a second recombinant hepatitis B vaccine, Engerix-B (SmithKlineBeecham), was approved for use in the…
In 1989, McGill University researcher Dr. Bernard Belleau developed the antiviral drug 3TC (Lamivudine), which became a critical…
On Jul. 23, 1986, the Recombinant hepatitis B vaccine (Recombivax HB by Merck) was licensed. Using recombinant DNA…
In May 1986, the vaccine Recombivax HB, which protects against hepatitis B infection, was approved for marketing in…

In 1986, while teaching a graduate course at the University of Alberta, Dr. Tyrrell found clues that might…

On Sept. 1, 1984, with the enactment of P.L. 98-369 by the U.S. Congress, coverage under Part B…

In 1982, the first hepatitis B viral vaccines, developed by Merck and also by the Pasteur Institute, were…

In 1981, the U.S. Food and Drug Administration (FDA) approved a plasma-derived hepatitis B vaccine for human use….

In 1976, Baruch Samuel Blumberg from the Institute for Cancer Research in Philadelphia was awarded the Nobel Prize…
In 1974, the Expanded Programme on Immunization was created within World Health Organization (WHO) in response to poor…
In 1974, Stanford Medicine researcher William S. Robinson successfully isolated the genome of the hepatitis B virus, which is…

In 1971, the testing of donated blood for Hepatitis B surface antigen began. The hepatitis B virus was…

In 1971, the U.S. Centers for Disease Control and Prevention (CDC) discovered that hepatitis B is sexually transmitted….

On Jun. 12, 1966, the Serum Institute of India was founded in 1966 by Dr. Cyrus Poonawalla with…
In 1947, after almost 10 years of inquiry into the nature of this illness, F.O. MacCallum, a British…