Dynavax announced first participants dosed in phase 1 clinical evaluating Medicagoメs COVID-19 vaccine candidate
On Jul. 14, 2020, Dynavax Technologies announced that the first participants have been dosed in the Phase 1…

On Jul. 14, 2020, Dynavax Technologies announced that the first participants have been dosed in the Phase 1…

On May 12, 2020, Abbott announced the FDA issued an Emergency Use Authorization (EUA) for the company’s molecular…

On Apr. 16, 2020, Dynavax and Sinovac Biotech, a leading provider of biopharmaceutical products in China, announced a…
![Dynavax donated 10,000 doses of HEPLISAV-Bᆴ [Hepatitis B Vaccine (Recombinant), adjuvanted] vaccine to emergency healthcare providers](https://lifesciencehistory.com/wp-content/uploads/2024/05/dynavax_logo-3.png)
On Apr. 8, 2020, Dynavax Technologies announced it had donated 10,000 doses of HEPLISAV-B adult vaccine to help…
On Mar. 19, 2020, Gilead Sciences announced the U.S. Food and Drug Administration (FDA) had approved a supplemental…
On Mar. 13, 2020, Enanta Pharmaceuticals announced it had initiated a program to discover direct-acting antiviral drug candidates…
On Nov. 13, 2019, researchers at University of California San Diego (UCSD) School of Medicine announced that they…
On Nov. 6, 2019, Abbott announced that a team of its scientists identified a new subtype of the…
On Feb. 15, 2019, the U.S. Centers for Disease Control and Prevention (CDC) published ACIP recommendations for use….

On Dec. 26, 2018, Sanofi announced the U.S. Food and Drug Administration (FDA) had approved VAXELIS (Diphtheria and…

On Nov. 2, 2018, the Immunization Practices Advisory Committee (ACIP) published updated recommendations on use of hepatitis A…
On Apr. 20, 2018, the Centers for Disease Control and Prevention (CDC) published Immunization Practices Advisory Committee (ACIP)…

On Jan. 12, 2018, the U.S. Centers for Disease Control and Prevention (CDC) published updated Immunization Practices Advisory…

On Nov. 9, 2017, Dynavax Technologies announced that the U.S. Food and Drug Administration (FDA) had approved HEPLISAV-B…

On Sept. 15, 2017, the U.S. Centers for Disease Control and Prevention (CDC) published updated GamaSTAN S/D prescribing…

On Aug. 28, 2017, the American Academy of Pediatrics (AAP) issued policy stating that newborns should routinely receive…

On Feb. 10, 2017, the U.S. Centers for Disease Control and Prevention (CDC) published the 2017 U.S. recommended…

On Sept. 13, 2016, Arbutus Biopharma announced that Dr. Michael J. Sofia, Arbutus’ Chief Scientific Officer, had been…

On Dec. 20, 2013, the U.S. Centers for Disease Control and Prevention (CDC) published guidance for HBV protection…

On Feb. 25, 2009, the Advisory Committee on Immunization Practices (ACIP) recommended routine hepatitis A vaccination for household…

On Oct. 27, 2008, the National Quality Forum included the hepatitis B birth dose among its consensus standards…

On Oct. 19, 2007, the U.S. Centers for Disease Control and Prevention (CDC) published updated recommendations for prevention…
On May 8, 2007, the National Cancer Institute (NCI) reported that people infected with the hepatitis C virus…
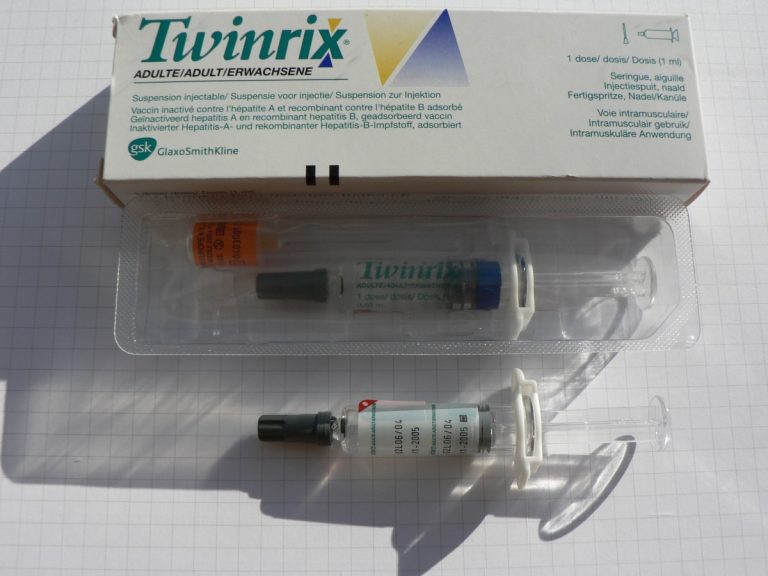
On Mar. 28, 2007, the U.S. Food and Drug Administration (FDA) announced that GlaxoSmithKline (King of Prussia, Pennsylvania)…

On Dec. 23, 2005, the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices…
On Oct. 17, 2005, the FDA approved lowering the age limit to 12 mos for the remaining U.S.-licensed…

On Aug. 11, 2005, the Food and Drug Administration (FDA) approved an application of a pediatric/adolescent formulation of…

On Dec. 13, 2002, the U.S. Food and Drug Administration (FDA) announced it had licensed a combined diphtheria…

On Dec. 3, 2002, Genentech drug Pegasys (peginterferon alfa-2a) was approved by the Food and Drug Administration (FDA)…

In 2002, Stanford geneticist Mark Kay uses a gene-therapy technique known as RNA inhibition to switch off genes…