Google DeepMind launches AI tool to help identify genetic drivers of disease
On Jan. 28, 2026, researchers at Google DeepMind have unveiled their latest artificial intelligence tool and claimed it…

On Jan. 28, 2026, researchers at Google DeepMind have unveiled their latest artificial intelligence tool and claimed it…
On Jan. 22, 2026, researchers backed by a Knight Initiative for Brain Resilience Catalyst Momentum Award have laid out…

On Dec. 11, 2025, the California Institute for Regenerative Medicine (CIRM) governing board approved funding of over $160…
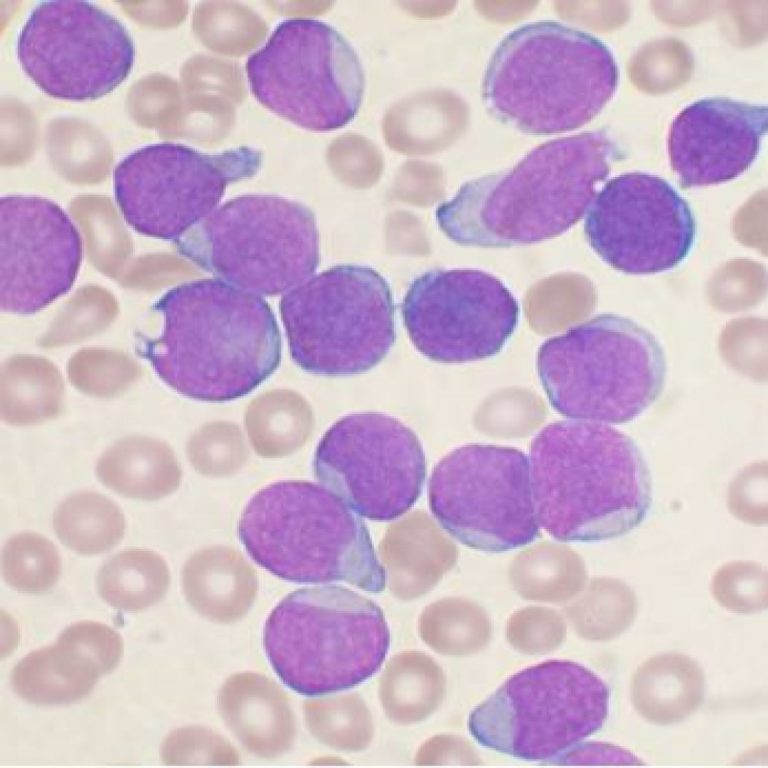
On Dec. 9, 2025, researchers at the UCLA Health Jonsson Comprehensive Cancer Center announced they have identified a…

On Nov. 24, 2025, a new AI model called popEVE developed by Harvard Medical School researchers and colleagues…

On Nov. 18, 2025, Arrowhead Pharmaceuticals announced that the U.S. Food and Drug Administration (FDA) has approved REDEMPLO…
On Nov. 17, 2025, in a major step forward for cancer care, researchers at ChristianaCare’s Gene Editing Institute…
On Nov. 10, 2025, a study led by the University of Liverpool reported that existing evidence does not…

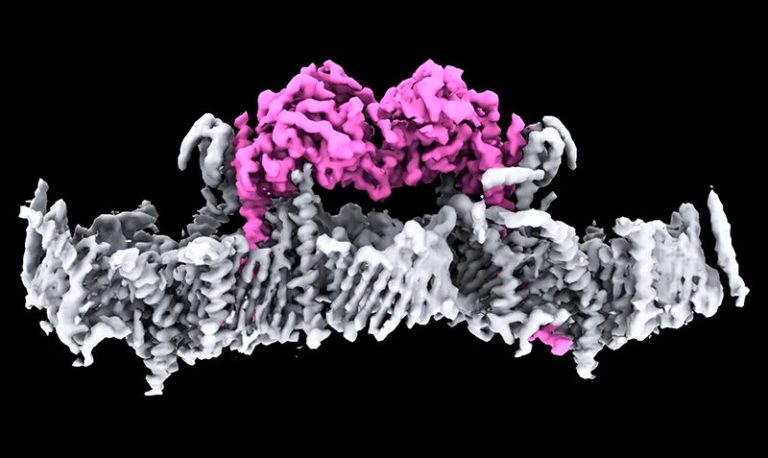
On Nov. 6, 2025, scientists from Oxford’s Radcliffe Department of Medicine have achieved the most detailed view yet…
On Oct. 31, 2025, investigators from Mass General Brigham, the Broad Institute of MIT and Harvard, and VA…

On Oct. 30, 2025, the California Institute for Regenerative Medicine (CIRM) approved awarding $27 million through three grants…

On Oct. 27, 2025, researchers at University of California San Diego School of Medicine (UC San Diego) have…

On Oct. 20, 2025, The Jackson Laboratory (JAX), a global leader in genetics and genomic medicine, announced it…

On Oct. 15, 2025, Broad Clinical Labs, in collaboration with Roche Sequencing Solutions and Boston Children’s Hospital, announced…
On Oct. 7, 2025, researchers at Baylor College of Medicine and the Jan and Dan Duncan Neurological Research…

On Sept. 25, 2025, The California Institute for Regenerative Medicine (CIRM) has approved awarding more than $73 million…

On Sept. 24, 2025, the U.S. Fish and Wildlife Service (USFWS) in collaboration with partners, announced the birth…

On Sept. 19, 2025, researchers at the Wellcome Sanger Institute and the Institute of Evolutionary Biology, Barcelona reported…
On Sept. 4, 2025, a global collaboration that includes experts at the Wellcome Sanger Institute announced mapping out…

On Aug. 27, 2025, scientists from the Wellcome Sanger Institute reveal insights into how our genes influence the…

On Aug. 11, 2025, University of Washington (UW) researchers announced the results of testing the efficacy of several…

On Jul. 24, 2025, a team of researchers from TGen, part of City of Hope, and Baylor Scott…
On Jul. 16, 2025, eight healthy babies were born in Britain with the help of an experimental technique…

On Jul. 12, 2025, an international team of geneticists and AI experts are adding whole new chapters to…

On Jul. 8, 2025, Colossal Biosciences announced the moa as its next de-extinction focus, continuing its mission to…

On Jul. 2, 2025, researchers from the Francis Crick Institute and Liverpool John Moores University (LJMU) announces they…

On Jul. 1, 2025, Anne Wojcicki’s bid to buy 23andMe, the genetic testing company she cofounded nearly 20…

On Jun. 30, 2025, as in something out of science fiction, a team of researchers reached into the…

On Jun. 26, 2025, a team of UK-based scientists announced they are developing technology to create the first…

On Jun. 25, 2025, Google Deepmind introduced AlphaGenome, a new artificial intelligence (AI) tool that more comprehensively and…