New guidelines announced to improve cystic fibrosis screening
On Apr. 3, 2025, The International Journal of Neonatal Screening, an international, peer-reviewed journal focused on newborn screening…

On Apr. 3, 2025, The International Journal of Neonatal Screening, an international, peer-reviewed journal focused on newborn screening…

On May 30, 2024, the California Institute for Regenerative Medicine (CIRM) announced it had awarded $53 million to…

On Nov. 23, 2023, Vertex announced that the European Medicines Agency (EMA) had validated a Type II variation…

On May 3, 2023, Vertex announced the Food and Drug Administration (FDA) ha approved KALYDECOᆴ (ivacaftor) for use…

On Apr. 26, 2023, Vertex announced the Food and Drug Administration (FDA) had approved the expanded use of…

On Jun. 9, 2021, Vertex Pharmaceuticals announced the U.S. Food and Drug Administration (FDA) approved expanded use of…

On Jan. 31, 2012, Vertex Pharmaceuticals announced that the U.S. Food and Drug Administration (FDA) had approved KALYDECOTM…
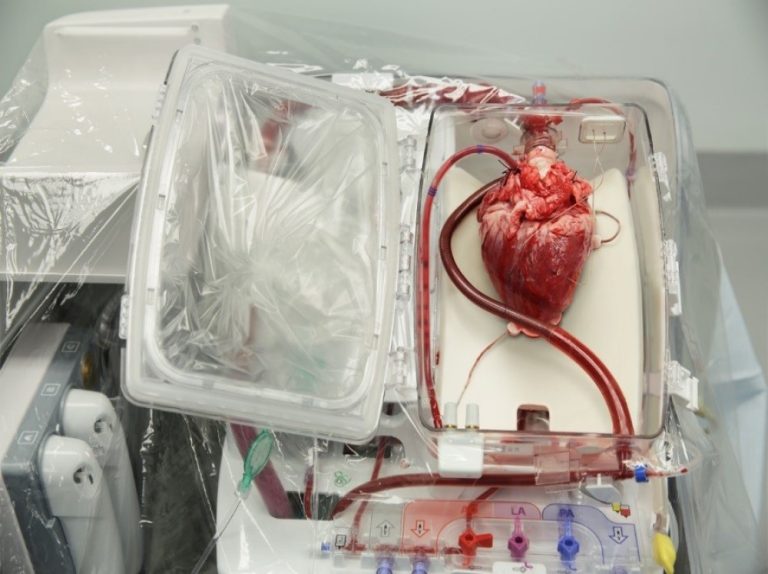
On Aug. 16. 2007, surgeons at the University of Washington (UW) Medical Center performed Seattle’s first adult heart-lung…

On Jan. 12, 2001, Corus Pharma was incorporated as a private venture-backed development stage biopharmaceutical company focused on…

On Sept. 22, 2000, Pathogenesis was acquired by Emeryville, California-based Chiron for $660 million or $38.50 per share….

In 1998, the Cystic Fibrosis Research Center was founded at University of Iowa’s College of Medicine. Today, the…

On Dec. 30, 1993, the Genentech drug Pulmozyme (dornase alfa) was approved by the U.S. Food and Drug…

On Sept. 3, 1992, PathoGenesis Corp. was founded by Robert Nowinski. The company, based in Seattle, developed an…

In 1989, CFTR, the gene causing cystic fibrosis was discovered by researcher Lap-Chee Tsui at Toronto’s Hospital for…

On Oct. 11, 1985, scientists in Canada and Massachusetts reported discovering the first genetic marker for the widespread…