FDA approved Genentech’s Avastin plus chemotherapy for treatment of advanced cervical cancer
On Aug. 14. 2014, Genentech announced that the U.S. Food and Drug Administration (FDA) had approved Avastin (bevacizumab)…
On Aug. 14. 2014, Genentech announced that the U.S. Food and Drug Administration (FDA) had approved Avastin (bevacizumab)…
On Feb. 28, 2014, the U.S. Centers for Disease Control and Prevention (CDC) published Immunization Practices Advisory Committee…
On Jun. 8, 2012, the U.S. Food and Drug Administration (FDA) announced that Genentech’s drug Perjeta (pertuzumab) was…

On Feb. 27, 2012, the Duke Cancer Institute opened a new 267,000-square-foot, $235 million Cancer Center that included…
On Sept. 14, 2008, the National Cancer Institute (NCI) researchers and colleagues have shown that increased expression of…
On Jan. 23, 2007, results from a large phase III clinical trial show that adult patients with previously…

In 2006, the National Cancer Institute (NCI) launched the TAILORx trial to determine whether gene expression patterns in…

On Dec. 7, 2005, results from several studies presented at the San Antonio Breast Cancer Symposium validated that…

On Apr. 27, 2005, results from two large National Cancer Institute sponsored randomized clinical trials showed that patients…

On Apr. 25, 2005, the combination of the targeted agent trastuzumab (Herceptin) and standard chemotherapy cuts the risk…

In Jan. 2005, the U.S. Food and Drug Administration (FDA) approved an albumin-stabilized nanoparticle formulation of paclitaxel (Abraxane)…

On Dec. 15, 2004, Amgen announced that following priority review, the FDA has approved Kepivance(TM) (palifermin), the first…
On Dec. 10, 2004, a National Cancer Institute (NCI) supported study determined that a new molecular test done…

On Nov. 18, 2004, the U.S. Food and Drug Administration (FDA) announced it had approved Genentech’s drug Tarceva…
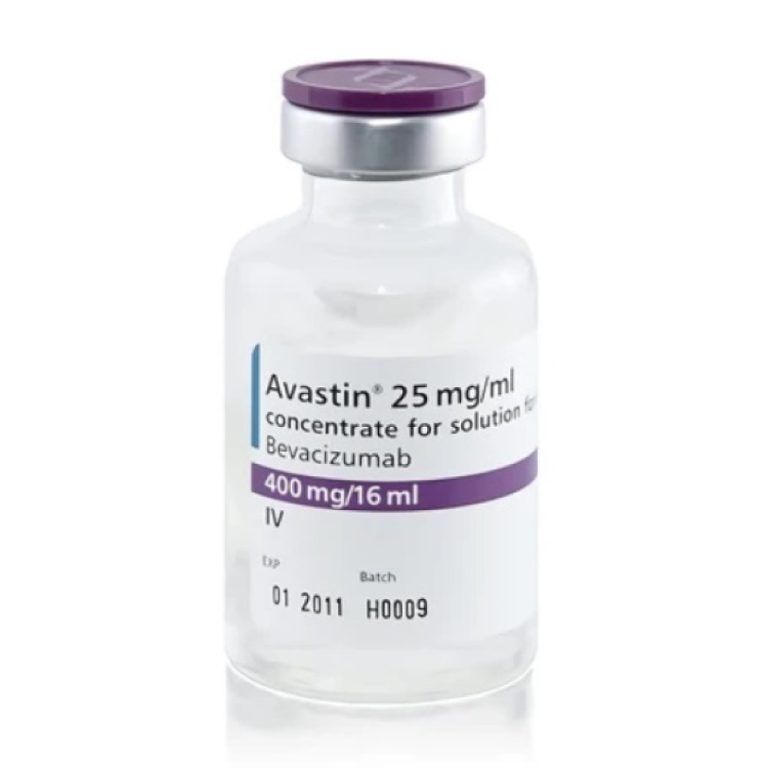
On Feb. 26, 2004, the U.S. Food and Drug Administration (FDA) announced it had approved the first antiangiogenic…
On Feb. 1, 2004, the U.S. Food and Drug Administration (FDA) announced it had approved Cetuximab, a recombinant…

On Dec. 12, 2002, the National Cancer Institute (NCI) announced that a clinical trial had shown that reducing…

On Jul. 22, 2002, Amgen announced that the U.S. Food and Drug Administration (FDA) had approved Aranesp (darbepoetin…

On Jun. 19, 2002, National Cancer Institute (NCI) scientists used microarray technology to determine the patterns of genes…

In 2000, researchers discovered that the most common form of non-Hodgkin lymphoma (NHL), diffuse large B-cell lymphoma, is…
On Sept. 25, 1998, the U.S. Food and Drug Administration (FDA) approved the monoclonal antibody Herceptin (Trastuzumab) for…

In 1996, Dr. Brenda Gallie identified the cause of drug-resistant childhood cancer of the retina, leading to an…
On Apr. 1, 1993, the FDA approved Amgen’s Epogen/Procrit for the treatment of anemia due to the effects…

On Mar. 4, 1991, researchers reported that post-operative (adjuvant) radiation therapy and chemotherapy were found to improve survival…

On Jun. 1, 1989, the FDA approved Amgen’s Epogen/Procrit for the treatment of anemia associated with chronic renal…

On Mar. 3, 1989, the U.S. Food and Drug Administration (FDA) approved Bristol-Myers’ PARAPLATIN (carboplatin) for the treatment…
In 1983, additive solutions were shown to extend the shelf life of red blood cells (RBC) to 42…

On Jul. 9, 1980, Dr. Vincent T. DeVita, Jr. became the ninth director of the National Cancer Institute…

In 1976, Bristol-Myers introduced CEENU (lomustine), a chemotherapy product for brain cancer and Hodgkins lymphoma. Lomustine is an…
In 1975, Dr. E. Donnall Thomas, a pioneer in bone marrow transplantation, secured a permanent treatment home at…