Karmanos Cancer Institute First in the World to use FDA-approved Radiofrequency Electromagnetic Device to Treat Liver Cancer
On Dec. 18, 2024, the Barbara Ann Karmanos Cancer Institute announced that the new TheraBionic P1 device, an…

On Dec. 18, 2024, the Barbara Ann Karmanos Cancer Institute announced that the new TheraBionic P1 device, an…

On Dec. 12, 2024, a study co-lead by researchers from the University of British Columbia, Vancouver General Hospital…
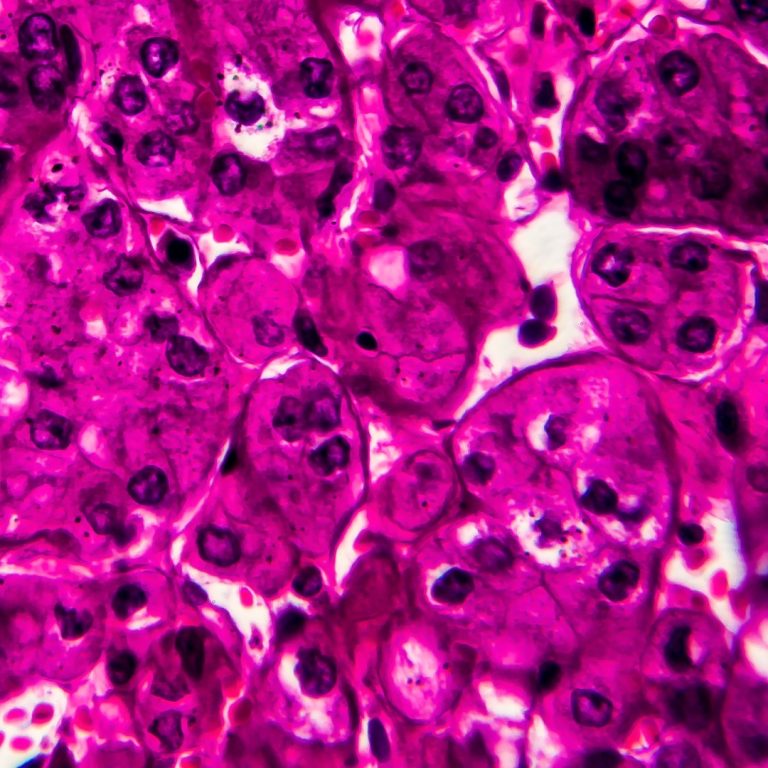
On Oct. 29, 2024, a study led by investigators from the UCLA Health Jonsson Comprehensive Cancer Center (UCLA…

On Apr. 26, 2024, an international team of researchers led by the National Cancer Institute (NCI) released an…

On Apr. 18, 2023, the U.S. Preventive Services Task Force (USPSTF) published a final recommendation statement on screening…

On Dec. 16, 2022, the U.S. Food and Drug Administration approved Ferring Pharmaceuticals’ Adstiladrin (nadofaragene firadenovec-vncg), a non-replicating…

On Jun. 21, 2022, Anixa Biosciences announced the publication of a peer-reviewed journal article in Clinical and Experimental…

On Aug. 13, 2021, Merck announced that the U.S. Food and Drug Administration (FDA) had approved WELIREG, an…

On Apr. 16, 2021, the FDA approved Bristol-Myers Squibb’ s Opdivo (nivolumab), in combination with certain types of…

On Jun. 11, 2020, Bristol-Myers Squibb announced that Opdivo (nivolumab) was approved by the FDA for the treatment…

On Jun. 11, 2020, scientists announced the development of a new test that can help identify people who…

On Mar. 19, 2020, The UArizona Cancer Center announced it was awarded a five-year, $6.9 million prestigious Program…
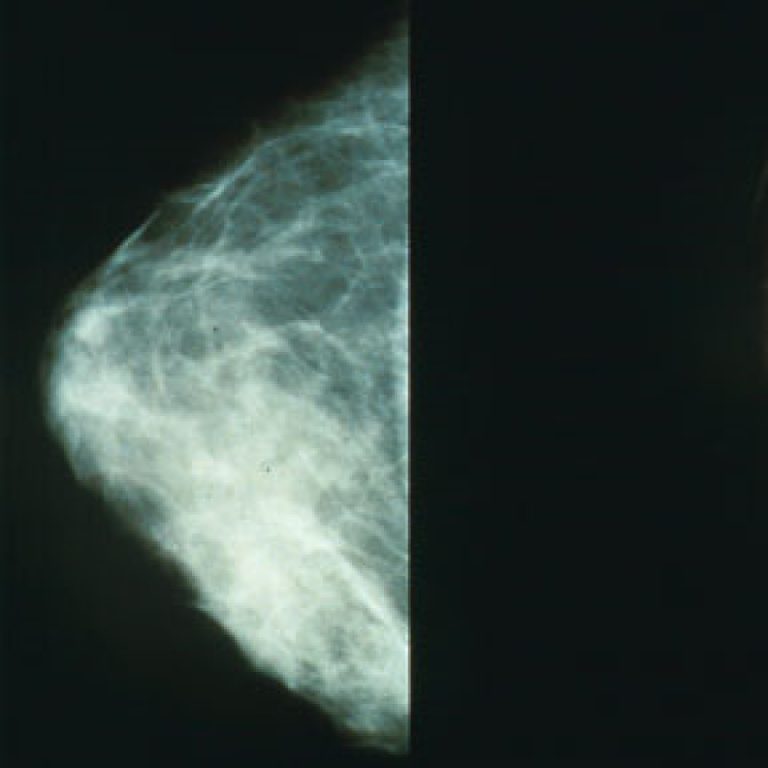
On Dec. 1, 2017, the U.S. Food and Drug Administration (FDA) approved Mylan’s Ogivri (trastuzumab-dkst) as a biosimilar…
On Aug. 15, 2016, Siteman Cancer Center received a $10.4 million five-year grant to Washington University researchers and…

On Sept. 10, 2014, an international team of scientists from Singapore, Thailand, China and Australia announced that they…
On Aug. 14. 2014, Genentech announced that the U.S. Food and Drug Administration (FDA) had approved Avastin (bevacizumab)…

On Jan. 30, 2012, the U.S. Food and Drug Administration (FDA) approved Genentech’s Erivedge (vismodegib) capsule, the first…

On Oct. 16, 2009, GlaxoSmithKline announced the U.S. Food and Drug Administration (FDA) had approved Cervarix [Human papillomavirus…

On Sept. 10, 2007, the National Cancer Institute (NCI) awarded a five-year, $12.5 million Specialized Program of Research…
On Oct. 10, 2006, Yale Cancer Center was awarded a SPORE (Specialized Programs of Research Excellence) grant for…

On May 19, 2002, researchers from the National Cancer Institute reported that the molecularly targeted drug bevacizumab slowed…
In 1999, Siteman Cancer Center investigators joined the STAR (Study of Tamoxifen and Raloxifene) trial for breast cancer…

In 1996, the American Cancer Society reported that the overall age-adjusted cancer mortality rate declined in each succeeding…

In 1996, Stanford Medicine developmental biologist Matthew Scott and a team at University of California, San Francisco discovered…
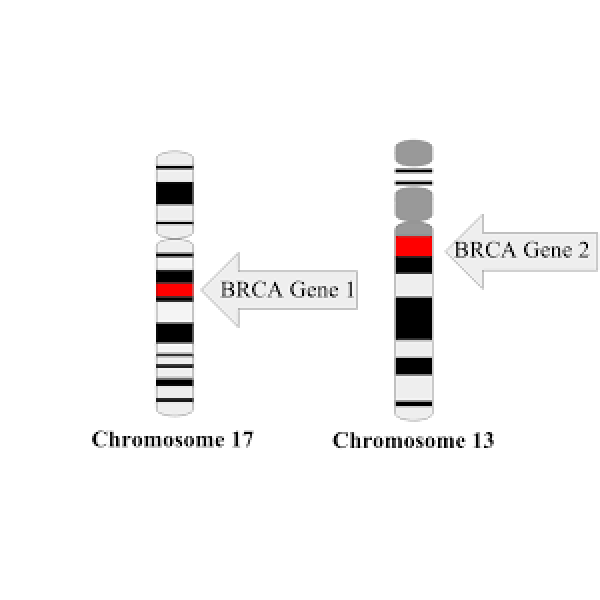
In 1995, the BRCA2 gene was mapped to chromosomal 13q. Just fifteen months later, Wooster et al. reported…
On Mar. 4, 1991, researchers reported that post-operative (adjuvant) radiation therapy and chemotherapy were found to improve survival…
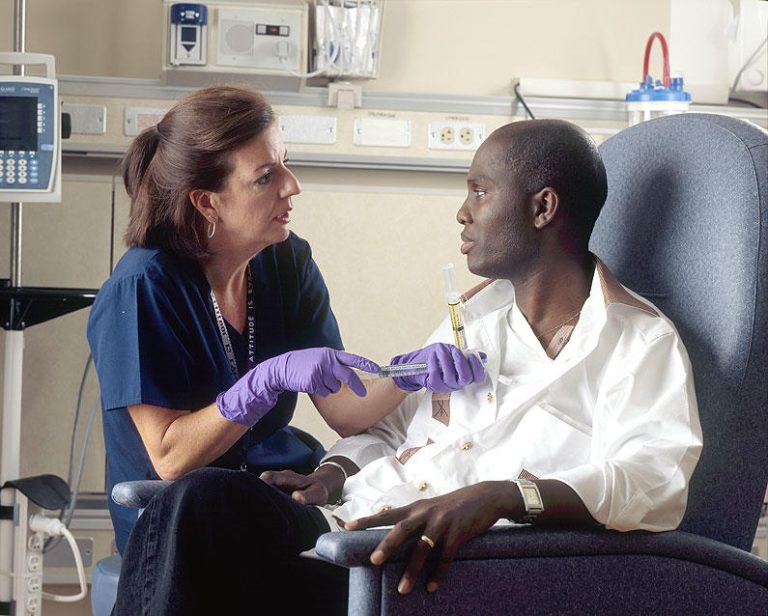
On Sept. 20, 1990, results from the first chemoprevention trial to show efficacy (vitamin A analogue against mouth…

On Mar. 3, 1989, the U.S. Food and Drug Administration (FDA) approved Bristol-Myers’ PARAPLATIN (carboplatin) for the treatment…
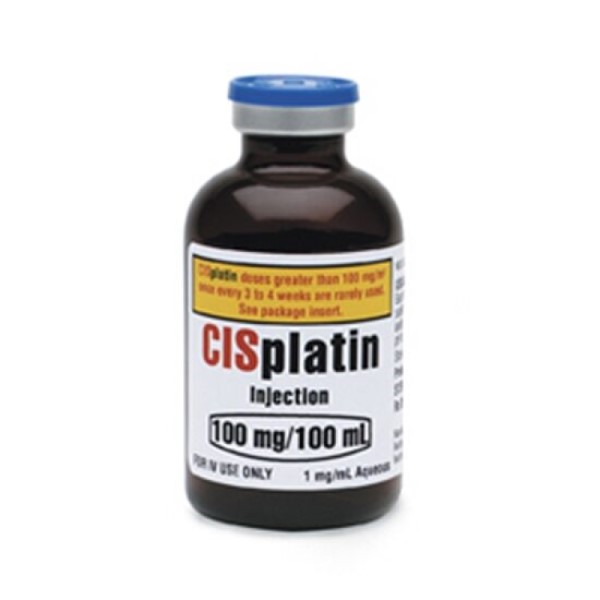
On Dec. 19, 1978, the U.S. Food and Drug Administration (FDA) approved cisplatin (Platinol) for use in combination…
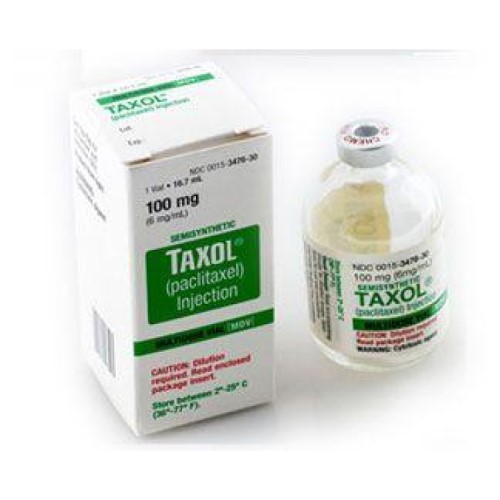
In 1976, Albert Einstein Cancer Center researchers identified the mechanism of action of Taxol, one of the most…