Intravacc in-licensed CimCure’s iBoost technology, expanding its vaccine platform portfolio
On Jul. 1, 2020, Intravacc announced the signing of a strategic partnership agreement with Dutch based CimCure, focusing…

On Jul. 1, 2020, Intravacc announced the signing of a strategic partnership agreement with Dutch based CimCure, focusing…

On Jun. 23, 2020, the FDA launched Project Patient Voice, an initiative of the FDAメs Oncology Center of…

On Jun. 12, 2020, the FDA announced it had approved an expanded indication for Merck’s GARDASIL9 for the…

On Jun. 11, 2020, scientists announced the development of a new test that can help identify people who…

On Jun. 10, 2020, African Americans who smoke are nearly 2.5 times more likely to have a stroke…

On Jun. 8, 2020, Innovent Biologics announced a strategic research and development collaboration with Roche covering multiple cell…

On Jun. 5, 2020, early data from a clinical study suggested that blocking the Bruton tyrosine kinase (BTK)…

On Jun. 4, 2020, vaccine manufacturers MSD, GSK, Innovax, Serum Institute of India and Walvax pledged to ramp…

On Jun. 2, 2020, Agenus announced the FDA’s clearance of AgenTus’ IND application for an allogeneic iNKT therapy….

On May 27, 2020, Gilead Sciences and Arcus Biosciences announced that the companies had entered into a 10-year…

On May 14, 2020, AIM ImmunoTech announced the FDA authorized the first human trial assessing the safety and…

On May 1, 2020, people with cancer who develop COVID-19 are much more likely to die from the…
On Apr. 29, 2020, a team of Texas A&M University researchers asked hundreds of frontline medical workers to…

On Apr. 27, 2020, Regeneron and Sanofi announced the primary endpoint of overall survival was met in a…
On Apr. 27, 2020, CTI BioPharma announced initiation of PRE-VENT, a Phase 3 study evaluating pacritinib in hospitalized…

On Apr. 14, 2020, AstraZeneca initiated a randomised, global clinical trial to assess the potential of Calquence (acalabrutinib)…

On Apr. 8, 2020, MediciNova announced that it had initiated a clinical trial of MN-166 (ibudilast) for acute…

On Apr. 9, 2020, Pfizer announced that the FDA had approved BRAFTOVIᆴ (encorafenib) in combination with cetuximab (marketed…

On Apr. 6, 2020, the National Cancer Institute awarded of $14.54 million to Roswell Park Comprehensive Cancer Center…

On Mar. 31, 2020, a team led by Pawel Kalinski, MD, PhD, of Roswell Park Comprehensive Cancer Center…

On Mar. 24, 2020, aTyr Pharma announced announced that the companyメs Hong Kong subsidiary, Pangu BioPharma, together with…

On Mar. 24, 2020, the Harold C. Simmons Comprehensive Cancer Center at UT Southwestern announced it had joined…

On Mar. 23 2020, The Priestley Medal was awarded to JoAnne Stubbe by the American Chemical Society “to…
On Mar. 17, 2020, Todos Medical announced a non-exclusive distribution agreement with 3D Biomedicine Science & Technology, a…
On Mar. 16, 2020, the Hormel Institute, University of Minnesota announced published research concerning remnants of ancient viruses…
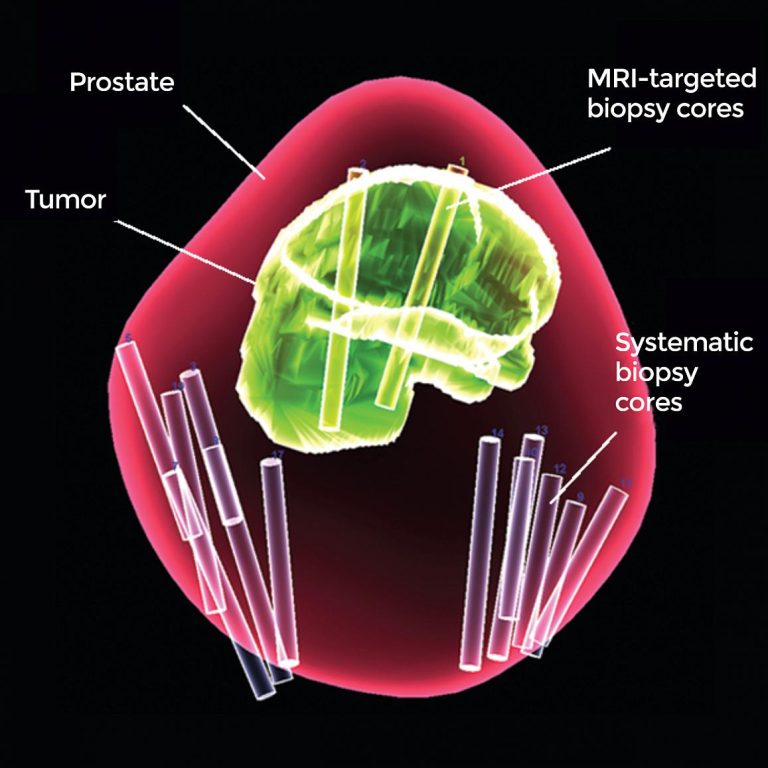
On Mar. 4, 2020, a method of testing for prostate cancer developed at the National Cancer Institute (NCI)…

On Mar. 2, 2020, Gilead Sciences announced it had acquired Forty Seven for $95.50 per share in cash…

On Feb. 20, 2020, Tim Boyle, CEO of Columbia Sportswear, and his wife, Mary, made a $10 million…
On Feb. 19, 2020, the Cancer Prevention & Research Institute of Texas (CPRIT) announced it had awarded 55…

On Feb. 13, 2020, a study published in Cell provided an unprecedented look at the dozens of molecular…