UTSW researchers identified new gene involved in breast cancer growth
On Jan. 12, 2021, a team researchers from the University of Texas Southwestern (UTSW) announced they had identified…
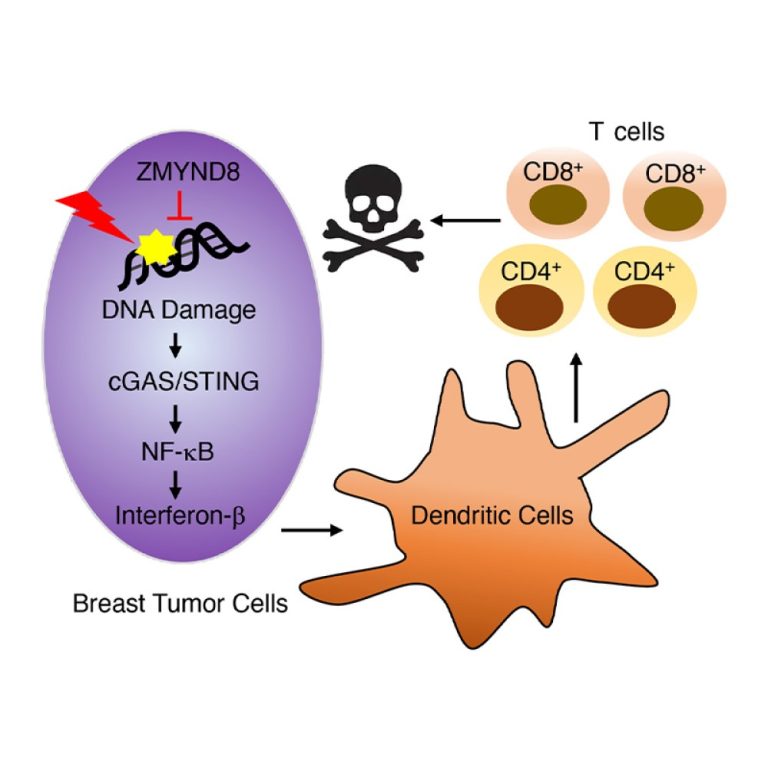
On Jan. 12, 2021, a team researchers from the University of Texas Southwestern (UTSW) announced they had identified…

On Nov. 13, 2020, Merck announced that the U.S. Food and Drug Administration (FDA) had approved KEYTRUDA, Merck’s…

On Nov. 13, 2020, Agilent Technologies announced it had received U.S. Food and Drug Administration (FDA) approval for…

On Nov. 4, 2020, McGill University Professor William Foulkes awarded 2020 Wilder-Penfield Prize for research in the genetics…

On Oct. 28, 2020, Seagen announced the closing of a $1.0 billion equity investment by Merck in 5.0…

On Sept. 9, 2020, NanoString Technologies announced the formation of the GeoMx Translational Leadership Network (GTLN). The GTLN…
On Aug. 24, 2020, a published study led by the Universities of Oxford and Birmingham found that, compared…

On Aug. 3, 2020, Todos Medical announced a partnership with PATHNOVA, a Singapore-based clinical laboratory, for the Company’s…

On Apr. 22, 2020, the U.S. Food and Drug Administration (FDA) granted accelerated approval to Trodelvy (sacituzumab govitecan-hziy),…

On Apr. 17, 2020, as part of Project Orbis, the U.S. Food and Drug Administration (FDA) approved Seagen’s…

On Dec. 20, 2019, the U.S. Food and Drug Administration (FDA) granted accelerated approval to Daiichi Sankyo’s Enhertu…

On Dec. 3, 2019, the Cold Spring Harbor Laboratory researchers with colleagues at UConn Health and Jackson Laboratory…

On Aug. 15, 2019, Masonic Cancer Center researchers Reuben Harris, PhD and Douglas Yee, MD announced that they…
On Feb. 28, 2019, the U.S. Food and Drug Administration (FDA) approved trastuzumab and hyaluronidase-oysk injection,for subcutaneous use…

On Jan. 21, 2019, researchers at Fred Hutchinson Cancer Research Center announced finding a way to essentially smother…
On Aug. 24, 2018, the Cancer Prevention and Research Institute of Texas (CPRIT) announced it passed a $2…

On Jun. 3, 2018, findings were reported from the groundbreaking Trial Assigning Individualized Options for Treatment (Rx), or…

On Mar. 9, 2018, Seattle Genetics announced it had completed its acquisition of Cascadian Therapeutics for approximately $614…

On Mar. 6, 2018, the U.S. Food and Drug Administration authorized 23andMe’s Personal Genome Service Genetic Health Risk…
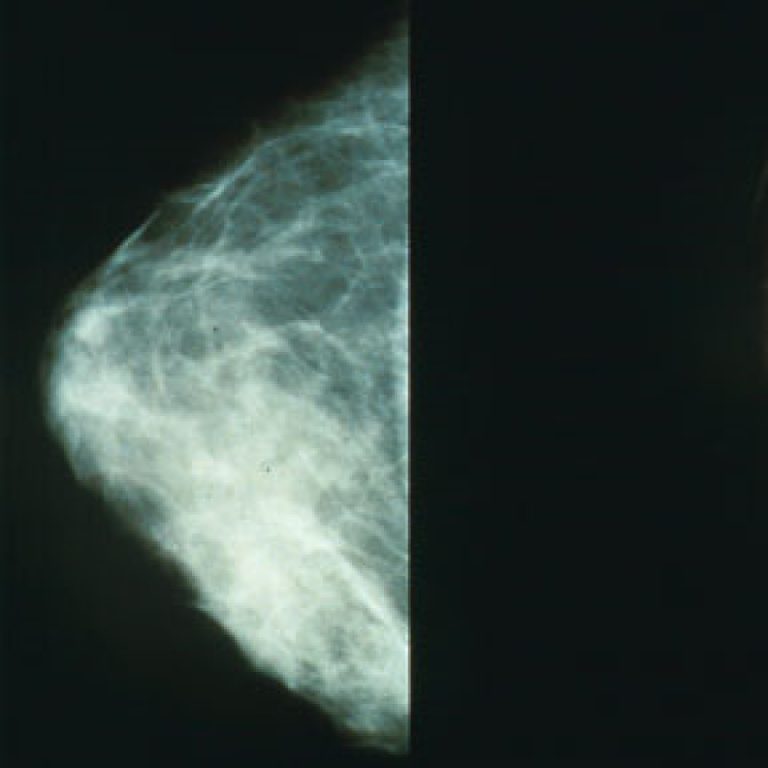
On Dec. 1, 2017, the U.S. Food and Drug Administration (FDA) approved Mylan’s Ogivri (trastuzumab-dkst) as a biosimilar…

On Jul. 17, 2006, the SERM drug raloxifene (Evista) was found to reduce breast cancer risk for postmenopausal…

On Jan. 11, 2016, the U.S. Preventive Services Task Force (USPSTF) recommended biennial screening mammography for women aged…

On Jul. 1, 2014, Genentech entered into an agreement to acquire Seragon Pharmaceuticals, a privately held biotechnology company…

On Feb. 22, 2013, the U.S. Food and Drug Administration (FDA) announced they had licensed ado-trastuzumab emtansine (Kadcyla)…

On Jun. 8, 2012, the U.S. Food and Drug Administration (FDA) announced that Genentech’s drug Perjeta (pertuzumab) was…

On May 13, 2008, the U.S. Congress passed the Breast Cancer and Environmental Research Act which amended the…

On Apr. 29, 2008, National Cancer Institute (NCI) researchers identified a pattern of gene activity in mice that…

In 2008, the Seattle Cancer Care Alliance introduced the Mammovan, making digital mammography more accessible to women throughout…
On Dec. 12, 2007, the Breast Cancer Research Stamp Reauthorization Act extended through Dec. 31, 2011, provisions requiring…

On Nov. 27, 2007, a new National Cancer Institute (NCI) model for calculating invasive breast cancer risk, called…