BriaCell Reports Sustained Complete Resolution of Lung Metastasis in Bria-OTS(TM) Patient
On Jan. 13, 2026, BriaCell Therapeutics announces the durable and sustained complete resolution of a lung metastasis in…

On Jan. 13, 2026, BriaCell Therapeutics announces the durable and sustained complete resolution of a lung metastasis in…

On Jan. 13, 2026, The American Cancer Society (ACS) released Cancer Statistics, 2026, the organization’s annual report on cancer facts and trends. The findings show, for…
On Nov. 19, 2025, the governing board of the Cancer Prevention and Research Institute of Texas (CPRIT) approved…

On Oct. 18, 2025, GRAIL announced that positive performance and safety results from its registrational PATHFINDER 2 study…

On Jul. 7, 2025, Celltrion announced that STOBOCLO® (denosumab-bmwo) and OSENVELT® (denosumab-bmwo), biosimilars referencing PROLIA® (denosumab) and XGEVA® (denosumab)…

On Apr. 21, 2025, the National Institutes of Health (NIH) reported that overall death rates from cancer declined steadily among…
On Apr. 11, 2025, the UK National Institute for Health and Care Excellence (NICE) recommended capivasertib (also called…
On Jan. 10, 2025, researchers at Roswell Park Comprehensive Cancer Center have identified a previously unknown cause of…

On Jan. 3, 2025, the U.S. General Dr. Vivek Murthy released a new Surgeon General’s Advisory on Alcohol…

On Nov. 8, 2024, Anixa Biosciences announced updated positive data from the Phase 1 clinical trial of its…
On Oct. 10, 2024, Genentech announced today that the U.S. Food and Drug Administration (FDA) approved ItovebiTM (inavolisib),…

On Oct. 1, 2024, the American Cancer Society (ACS) released Breast Cancer Statistics, 2024, the organization’s biennial update…

On Sept. 24, 2024, researchers at the National Institutes of Health (NIH) reported results of a study that…

On Sept. 18, 2024, researchers from the Wellcome Sanger Institute and their collaborators reported that they had identified…
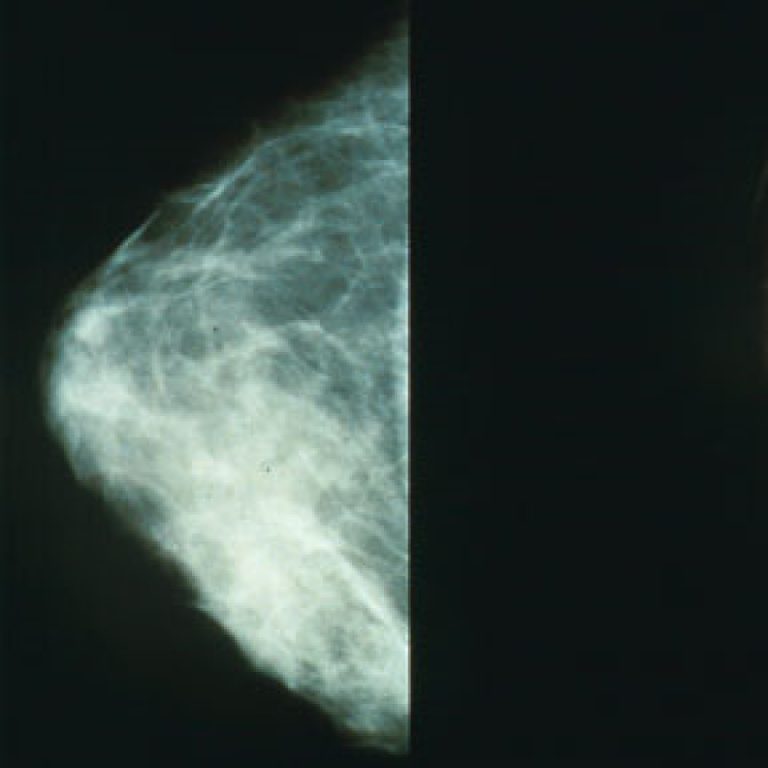
On Sept. 3, 2024, a major study led by the UC Davis Comprehensive Cancer Center (UC DAVIS) has…

On Sep. 3, 2024, the National Cancer Institute (NCI) announced that the latest Cancer Trends Progress Report included…

On Jul. 31, 2024, a large study led by researchers at the American Cancer Society (ACS) suggested incidence…

On Jun. 26. 2024, the World Health Organization reported that new data show that nearly one third (31%)…

On May 15, 2024, the American Association for Cancer Research (AACR) released its biennial Cancer Disparities Progress Report…

On Apr. 30, 2024, the U.S. Preventive Services Task Force (USPSTF) recommended that all women get screened for…

On Dec. 6, 2023, Anixa Biosciences announced updated positive results from the Phase 1 clinical trial of its…

On Aug. 31, 2023, the U.S. Food and Drug Administration (FDA) cleared for marketing the updated 23andMe Personal…

On May 9, 2023, the U.S. Preventive Services Task Force (USPSTF) recommended that all women get screened for…
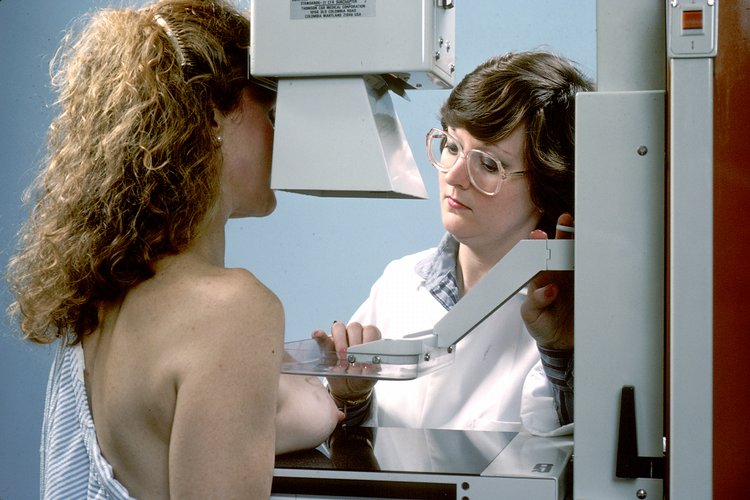
On Mar. 9, 2023, the U.S. Food and Drug Administration (FDA) announced that it had published updates to…

On Feb. 3, 2023, the World Health Organization (WHO) released an updated Global Breast Cancer Initiative (GBCI) Framework…

On Dec. 24, 2022, the U.S. Food and Drug Administration (FDA) approved updated labeling for Genentech’s capecitabine tablets…
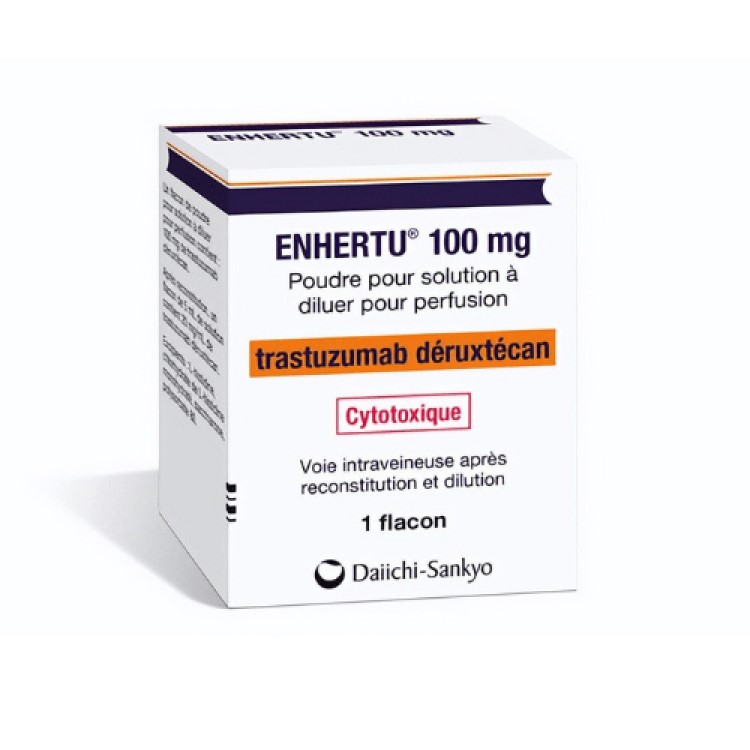
On Aug. 5, 2022, the U.S. Food and Drug Administration (FDA) approved Daiichi Sankyo’s Enhertu (fam-trastuzumab-deruxtecan-nxki), an IV…

On Jun. 21, 2022, Anixa Biosciences announced the publication of a peer-reviewed journal article in Clinical and Experimental…

On Apr. 6, 2022, a new study in the Journal of Clinical Oncology showed that women receiving certain…

On Oct. 26, 2021, Anixa Biosciences announced that, in conjunction with its partner, Cleveland Clinic, it had commenced…