Early signs of Parkinson’s can be identified in the blood
On Jan. 29, 2026, a team led by researchers at Chalmers University of Technology and Oslo University Hospital,…
On Jan. 29, 2026, a team led by researchers at Chalmers University of Technology and Oslo University Hospital,…

On Dec. 3, 2025, Massachusetts Institute of Technology (MIT) engineers announced they can accurately measure blood glucose by…

On Nov. 18, 2025, CSL, Australia’s largest biotech company announced plans to expand its US presence with a…

On Nov. 5, 2025, a large study of adults with type 2 diabetes showed that those who consistently…

On Oct. 13, 2025, the U.S. Food and Drug Administration (FDA) announces it has cleared the Elecsys pTau181…
On Oct. 13, 2025, University of Cambridge scientists announced they have used human stem cells to create three-dimensional…

On Sept. 16, 2024, researchers from the National Health Service Blood and Transplant (Bristol), NHSBT’s International Blood Group…

On Mar. 7, 2023, Abbott announced that it had received U.S. Food and Drug Administration clearance for what…

On Feb. 22, 2022, Washington University in St. Louis announced that a blood test developed at the University’s…

On Mar. 8, 2021, the National Institutes of Health (NIH) announced that it had launched the last of…

On Apr. 30, 2020, InBios announced that it had been awarded a contract from the Biomedical Advanced Research…

On Mar. 8, 2018, Sanofi acquired Waltham, Massachusetts-based Bioverativ for for $11.6 billion. Bioverativ’s extended half-life therapies, Eloctate…

On Mar. 2, 1985, the U.S. Food and Drug Administration (FDA) announced the approval of the Abbott first antibody…
In 1972, the Red Cross called for a national blood policy to support standardized practices and end paid…

In 1972, the Food and Drug Administration’s (FDA) new Bureau of Biologics began to regulate all 7000 U.S….

In 1950, physician Audrey Smith reported the use of glycerol cryoprotectant for red blood cells. During her work…

On Feb. 4, 1941, the Red Cross began a National Blood Donor Service to collect blood for the…
In 1940, the U.S. government established a national blood collection program. That same year the National Research Council…
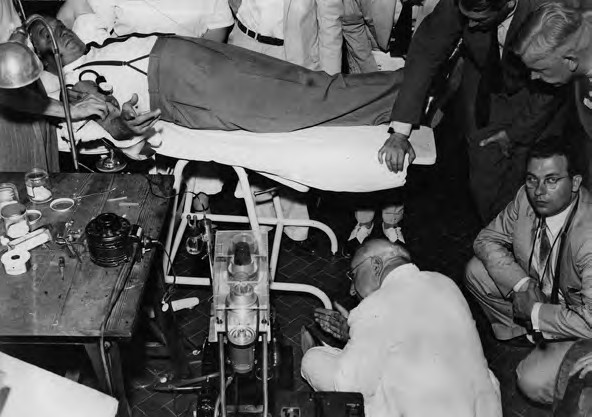
In 1940, Edwin Cohn, a professor of biological chemistry at Harvard Medical School, developed cold ethanol fractionation, the…

In 1940, Charles R. Drew, MD, an African American surgeon and Howard University researcher, began an early blood…

In 1938, University of Iowa researcher Elmer DeGowin developed the first reliable methods of preserving and shipping blood,…

On Jun. 15, 1667, the first successful blood transfusions from sheep to humans were reported separately by Jean-Baptiste…