Roche partnered with Moderna to include SARS-CoV-2 antibody test in ongoing COVID-19 vaccine trials
On Dec. 9, 2020, Roche announced a partnership with Moderna to utilise the Elecsys Anti-SARS-CoV-2 S antibody test…

On Dec. 9, 2020, Roche announced a partnership with Moderna to utilise the Elecsys Anti-SARS-CoV-2 S antibody test…

On Dec. 7, 2020, an universal influenza virus vaccine, which produces antibodies that target the part of the…

On Dec. 3, 2020, Moderna announced in a letter to the editor published in the New England Journal…
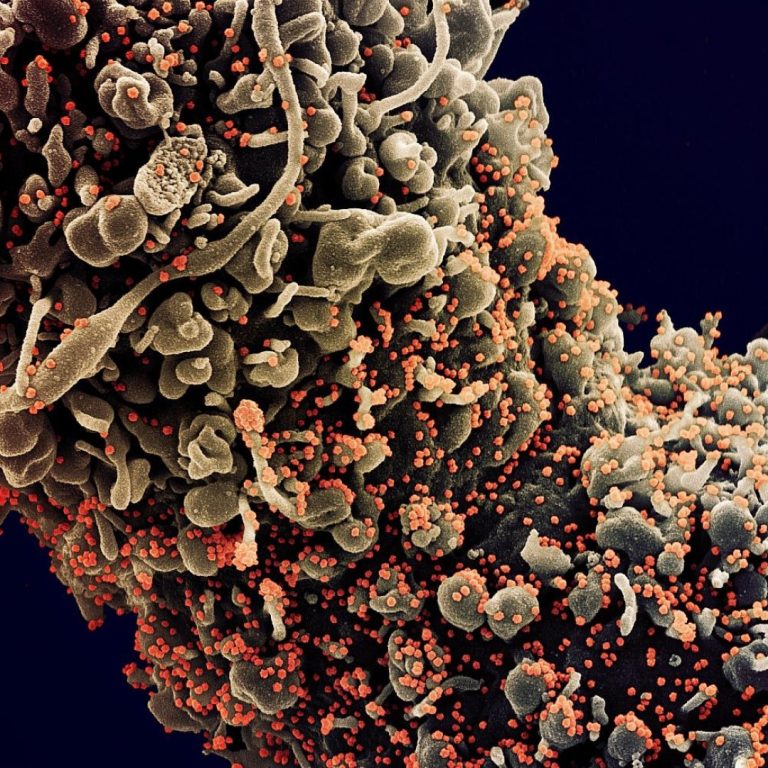
On Dec. 3, 2020, AXIM Biotechnologies announced the development and patent filing for an enzyme-linked immunosorbent assay (ELISA)-based…

On Dec. 2, 2020, Roche announced that its Elecsysᆴ Anti-SARS-CoV-2 S antibody test had received Emergency Use Authorization…

On Nov. 30, 2020, a City of Hope investigational vaccine produced strong protective immunity against SARS-CoV-2, including neutralizing antibodies…
On Nov. 24, 2020, JAMA reported that fewer than 10% of people in tye U.S. as of September…

On Nov. 19, 2020, XBiotech announced data for its breakthrough candidate therapy for treating infections of influenza and…

On Nov. 12, 2020 Vaxart announced additional results from its Hamster Challenge Study. The study evaluated Vaxartメs recombinant…

On Nov. 11, 2020, Howard Hughes Medical Institute Investigator Michel Nussenzweig and his team reported that people who…

On Nov. 9, 2020, PerkinElmer announced that EUROIMMUN, a PerkinElmer company, had launched the Anti-SARS-CoV-2 QuantiVacTM ELISA (IgG)…

On Nov. 6, 2020, the FDA authorized the first serology test that detected neutralizing antibodies from recent or…

On Oct. 31, 2020, the U.S. Department of Health and Human Services and the U.S. Department of Defense…

On Oct. 28, 2020, researchers at the University of Nebraska Medical Center (UNMC) reported the results of a…

On Aug. 27, 2020, immunologists at Scripps Research, in partnership with colleagues at the IAVI Neutralizing Antibody Center,…
On Oct. 22, 2020, IAVI, a nonprofit scientific research organization dedicated to addressing urgent, unmet global health challenges,…

On Oct. 21, 2020, ImmunityBio and NantKwest announced that the first patient had been dosed in the Phase…

On Oct. 19, 2020, Bio-Techne announced that ProteinSimple, a Bio-Techne brand, released its SARS-CoV-2 Multi-Antigen Serology Module for…

On Oct. 19, 2020, LabCorp announced a test that provided a quantitative measurement of an individualメs SARS-CoV-2 IgG…

On Oct. 19, 2020, Evotec announced that its Seattle-based subsidiary, Just – Evotec Biologics had received a grant…

On Oct. 15, 2020, ImmunityBio announced it had received authorization from the FDA to begin a Phase I…

On Oct. 14, 2020, the U.S. Department of Health and Human Services (HHS) and Department of Defense (DOD)…
On Oct. 14, 2020, the FDA approved Regeneron Pharmaceutical’s Inmazeb (atoltivimab, maftivimab, and odesivimab-ebgn), a mixture of three…

On Oct. 12, 2020, Thermo Fisher Scientific introduced two new SARS-CoV-2 antibody tests: the Thermo Scientific OmniPATH COVID-19…
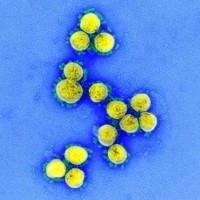
On Oct. 8, 2020, a clinical trial to test the safety, tolerability and efficacy of a combination treatment…
On Oct. 8, 2020, a clinical trial to test the safety, tolerability and efficacy of a combination treatment…

On Oct. 8, 2020, Emergent BioSolutions announced the initiation of the Phase 3 clinical trial that will evaluate…

On Oct. 8, 2020, Eli Lilly and the Bill & Melinda Gates Foundation, as part of the COVID-19…
On Oct. 7, 2020, Sorrento Therapeutics announced that it had discovered a small molecule termed Salicyn-30 that demonstrated…

On Oct. 7, 2020, Eli Lilly announced additional details on its SARS-CoV-2 neutralizing antibody programs ヨ including interim…