Fulgent Genetics launched at-home neutralizing antibody test for COVID-19
On Oct. 27, 2021, Fulgent Genetics announced that it had launched an antibody test for COVID-19 which specifically…

On Oct. 27, 2021, Fulgent Genetics announced that it had launched an antibody test for COVID-19 which specifically…

On Oct. 21, 2021, the National Institutes of Health announced that a booster dose of the mRNA-1273 COVID-19…

On Oct. 5, 2021, PerkinElmer announced that the U.S. Food and Drug Administration had provided Emergency Use Authorization…

On Sept. 15, 2021, the U.S. Department of Defense in coordination with the Department of Health and Human…

On Sept. 15, 2021, InBios announced that it had received Emergency Use Authorization from the U.S. Food and…

On Aug. 30, 2021, AXIMᆴ Biotechnologies announced that the Companyメs manufacturing partner Empowered Diagnostics had filed an amended…
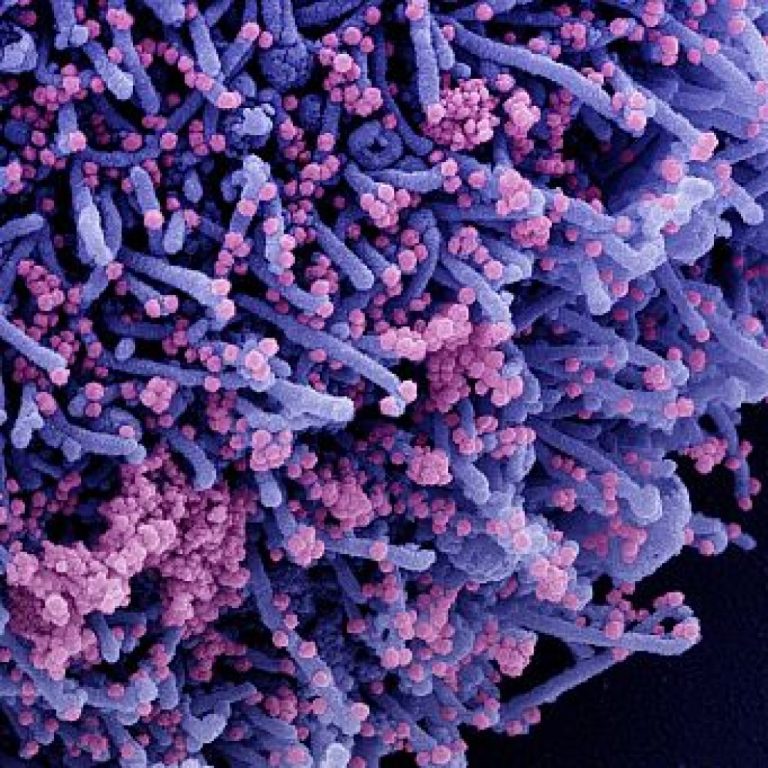
On Aug. 30, 2021, Oragenics announced that the stabilized pre-fusion spike protein trimer produced by its Canadian collaborator…

On Aug. 30, 2021, the Mayo Clinic reported that in an observational study the combination of casirivimab and…

On Aug. 27, 2021, the USDA’s National Veterinary Services Laboratories announced confirmation of SARS-CoV-2 (the virus that causes…

On Aug. 25, 2021, Johnson & Johnson announced data supporting the use of its COVID-19 vaccine as a…

On Aug. 20, 2021, Amyris announced promising in-vivo results in a study of its licensed RNA vaccine through…

On Aug. 19, 2021, Oregon Health & Science University (OHSU) announced that it was part of a randomized,…

On Aug. 17, 2021, researchers reported the delta variant of the virus that causes COVID-19 was not particularly…

On Aug. 16, 2021, CureVac and GSK announced publication on the preprint server bioRxiv of preclinical data investigating…

On Aug. 12, 2021, Moderna announced the publication of new data on the durability of the Moderna COVID-19…
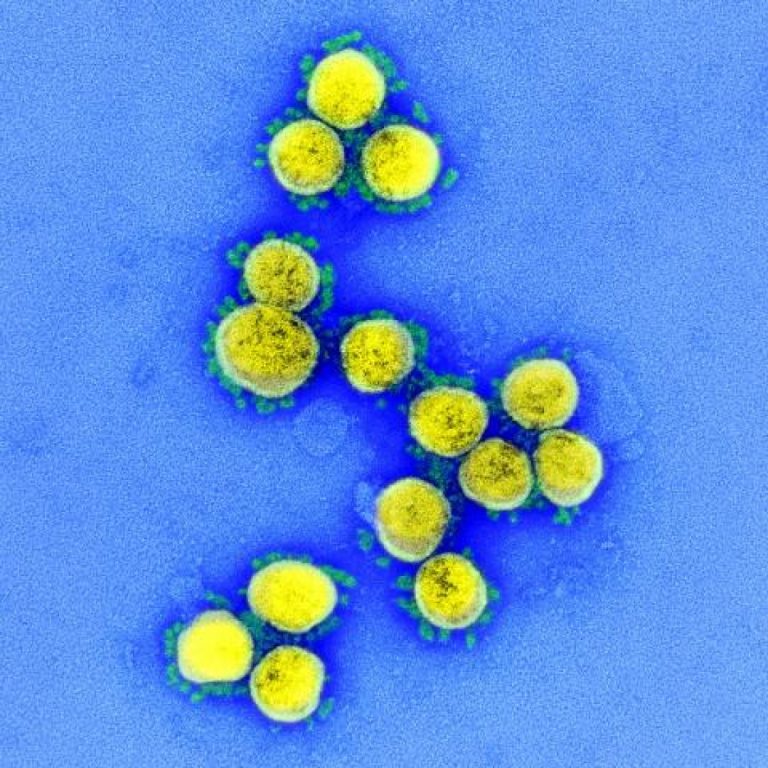
On Aug. 5, 2021, Novavax announced preliminary data demonstrating that a single booster dose of its recombinant nanoparticle…

On Jun. 28, 2021, researchers running the University of Oxford-led Com-COV study reported that alternating doses of the…

On Jun. 23, 2021, the National Institutes of Health announced that an observational study had begun to evaluate…

On Jun. 16, 2021, the University of Oxford announced the Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial demonstrated…
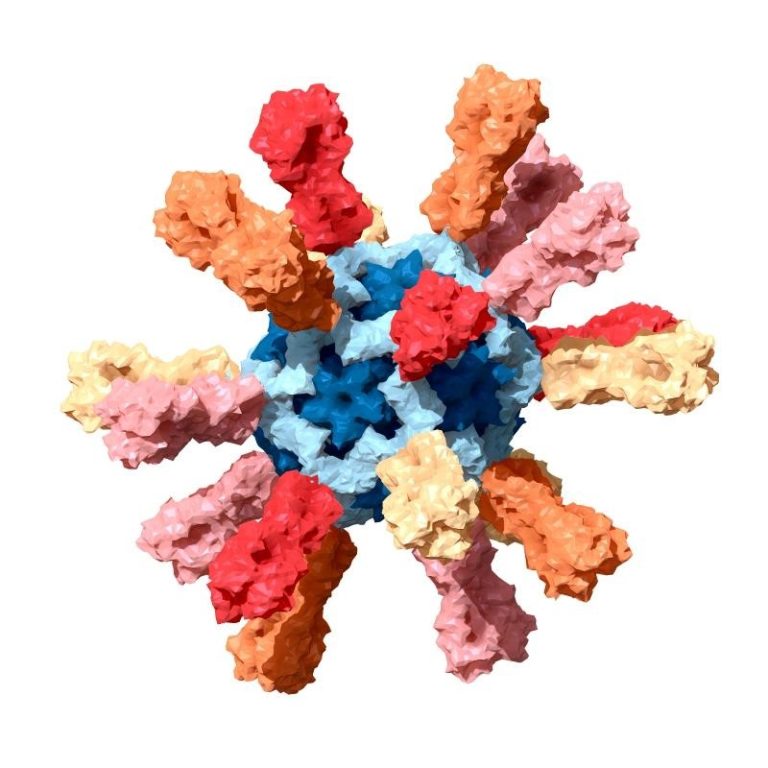
On Jun. 1, 2021, the National Institutes of Health (NIH) announced the first-in-human, Phase 1 trial assessing the…
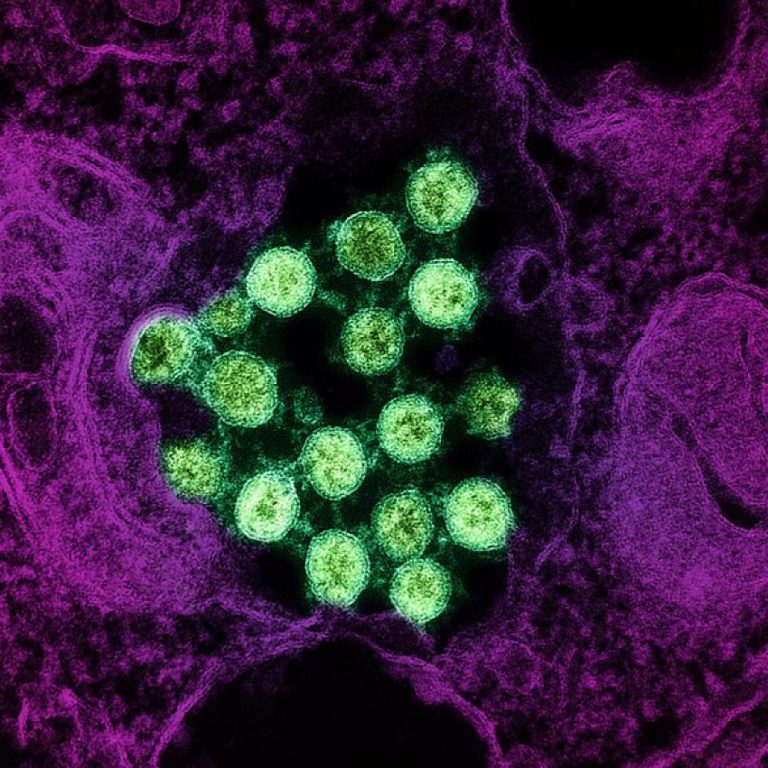
On May 27, 2021, Seattle Children’s researchers published a study that uncovered a deeper understanding of why people…

On May 24, 2021, LabCorp reported that nearly 87% of naturally infected COVID-19 patients maintained antibodies to SARS-CoV-2…

On May 12, 2021, InBios announced that it had received Emergency Use Authorization from the U.S. Food and…

On May 12, 2021, InBios announced that it had received Emergency Use Authorization from the U.S. Food and…

On May 12, 2021, Inovio Pharma announced that its next-generation Pan-COVID-19 vaccine candidate, INO-4802, induced potent neutralizing antibodies…

On May 3, 2021, Precipio announced that it had successfully launched its COVID-19 rapid antibody test (20 minute)…

On Apr. 7, 2021, Moderna announced publication of antibody persistence data out to 6 months following the second…

On Mar. 29, 2021, an international research team led by Oxford University scientists announced they had developed a…

On Mar. 24, 2021, AXIM Biotechnologies announced that partner Empowered Diagnostics, had filed an Emergency Use Authorization or…

On Mar. 19, 2021, Scientists at the University of Oxford released pre-print data measuring the level of antibodies…