Sabin Vaccine Institute Delivered Marburg Vaccines to Combat Outbreak in Rwanda
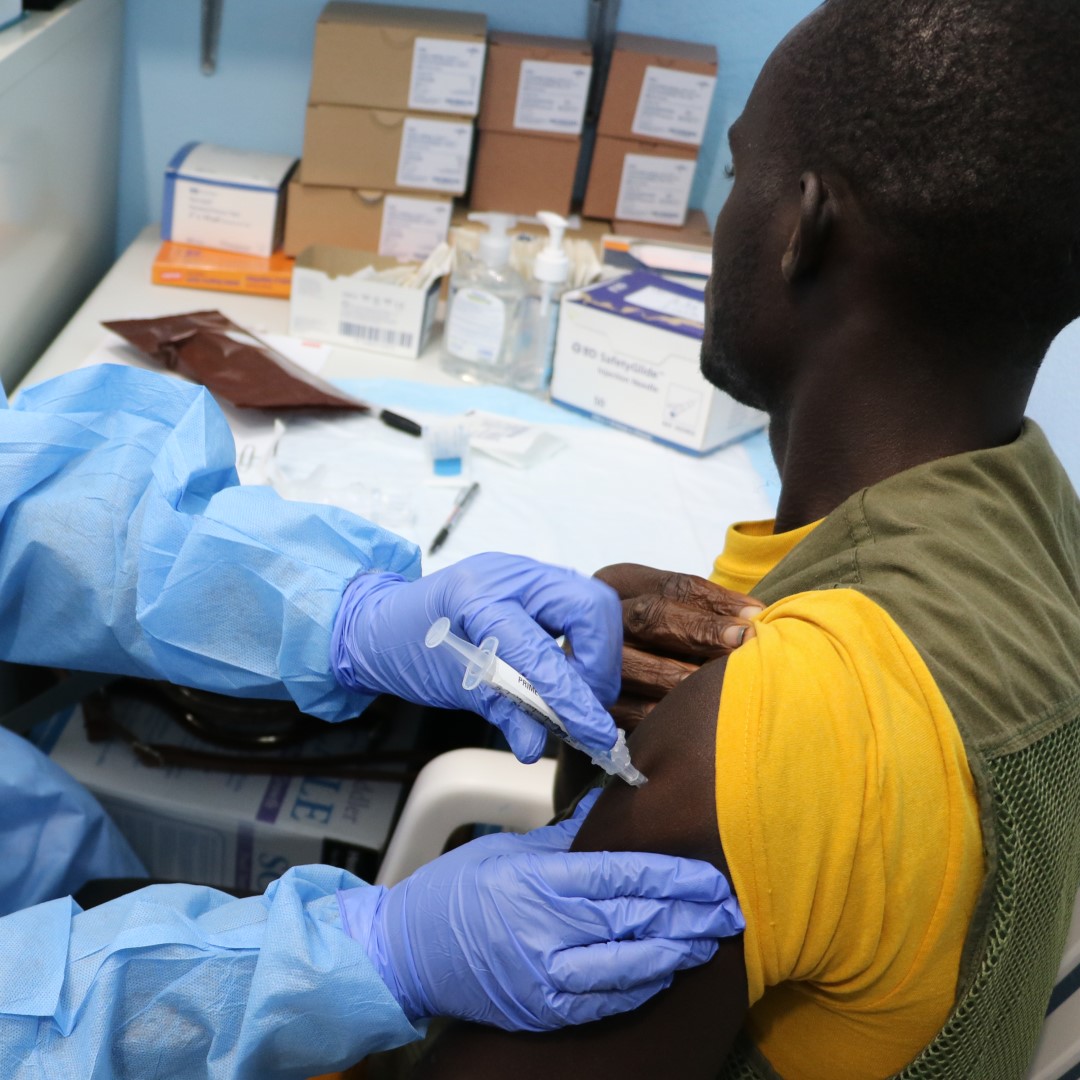
On Oct. 5, 2024, the Sabin Vaccine Institute announced it had provided its investigational Marburg vaccine to Rwanda to support the ongoing outbreak response. The initial shipment of approximately 700 vaccine doses was used in a trial targeting frontline workers, including healthcare professionals who have been hardest hit by the deadly virus.
Sabin had entered into a clinical trial agreement with the Rwanda Biomedical Centre, the trial sponsor, to provide investigational doses for the Phase 2 rapid response open-label study. Per the approved protocol, approximately 700 high-risk adults, starting with health care providers, will be dosed at 6 clinical trial sites in Rwanda. Pending a request from Rwandan officials and authorization from the U.S. Biomedical Advanced Research and Development Authority (BARDA), Sabin planned to supply additional vaccines.
To date, there are no licensed vaccines or treatments for Marburg, which has a mortality rate of up to 88%. Sabin’s single-dose vaccine, based on the cAd3 platform, is in Phase 2 trials in Uganda and Kenya with no safety concerns reported to date. Results from Phase 1 clinical trials and nonclinical studies indicate that the vaccine is safe and elicits rapid, robust immune responses.
Tags:
Source: Sabin Vaccine Institute
Credit:
