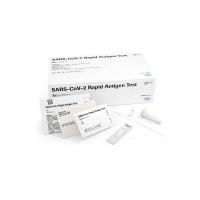
Roche COVID-19 at-home test granted FDA Emergency Use Authorization to expand access to rapid self-testing solutions in the United States
On Dec. 24, 2021, Roche announced that the U.S. Food and Drug Administration had granted Emergency Use Authorization for its COVID-19 At-Home Test.
The test uses a simple anterior nasal swab sample that can be conveniently self-collected and self-tested by individuals aged 14 years and older, and by an adult for children aged 2-13 years old. The test produced accurate, reliable and quick results in as few as 20 minutes for SARS-CoV-2 and all known variants of concern, including Omicron.
Tags:
Source: Roche
Credit:
