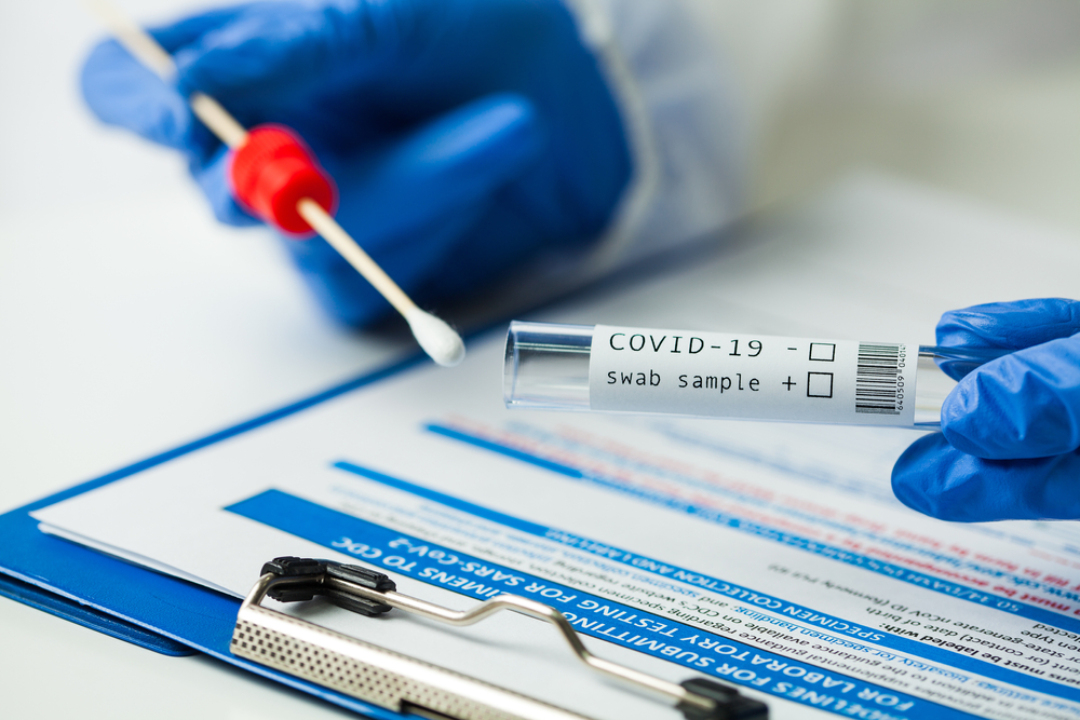
Quest Diagnostics launched three new combined COVID-19 and respiratory virus tests
On Sept. 30, 2020, Quest Diagnostics announced three different test options for healthcare providers across the U.S.to aid in the diagnosis of COVID-19 and differentiate it from other seasonal respiratory infections, including influenza.
Each test is designed to use a single specimen, promoting faster test ordering, reporting and diagnosis, as compared to ordering multiple tests, for potentially improved patient care. The tests may also help reduce demand for specimen collection devices amid supply constraints.
The new test options include the cobas® SARS-CoV-2 & Influenza A/B*, an automated high throughput multiplex real-time RT-PCR assay from Roche. The U.S. Food and Drug Administration (FDA) granted Emergency Use Authorization (EUA) for the test earlier this month.
The test is intended for simultaneous qualitative detection and differentiation of SARSCoV-2, the virus that causes COVID-19, and/or influenza A or B virus RNA from individuals suspected of respiratory viral infection consistent with COVID-19 by their healthcare provider.
The test may be performed on healthcare provider-collected nasal and nasopharyngeal swab specimens and self-collected nasal swab specimens (collected in a healthcare setting with instruction by a healthcare provider). Nasal swabs include anterior nares swabs, which involve collecting a specimen from the lower nostril.
Tags:
Source: Quest Diagnostics
Credit:
