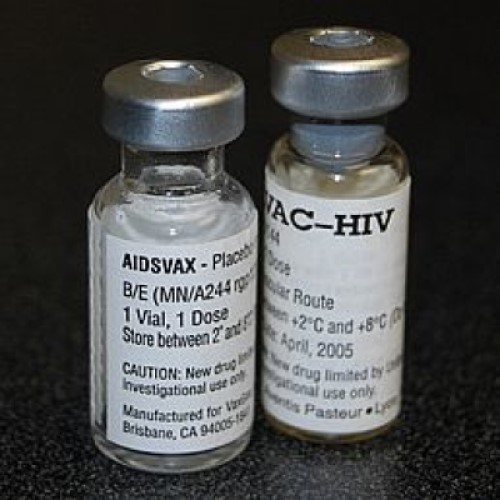
Phase III results for first HIV/AIDS (Aidsvax) vaccine reported
On Sept. 24, 2009, the second Phase III HIV-1 vaccine trial, also known as RV144, that began in Thailand in October 2003 was concluded. This test-of-concept trial was the largest HIV vaccine study ever conducted in humans, enrolling more than 16,000 Thai men and women from the general population.
The vaccine used in the RV144 trial is a combination of two vaccines given in a ‘prime–boost’ strategy: ALVAC-HIV from Sanofi Pasteur (Swiftwater, PA) and AIDSVAX from VaxGene (San Francisco, CA). Both vaccines were based on HIV-1 B and E clades that commonly circulate in Thailand. RV144 was designed to test the ability of this vaccine combination to prevent HIV infection, as well as its ability to reduce the level of HIV RNA in the blood of the vaccinees that became infected.
Despite skepticism and controversies about the ability of the combination of these vaccine modalities to induce sufficient immune responses, the vaccine regimen proved to be safe and demonstrated 31.2% efficacy in preventing HIV infection. While the results of this trial may suggest for the first time the feasibility of a vaccine for HIV, the limited level of protection from viral infection implies that more work will be needed to generate a vaccine that protects the general population from HIV acquisition.
Tags:
Source: National Library of Medicine
Credit:
