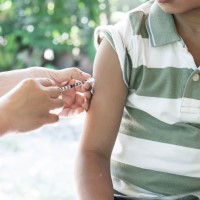
Pfizer and BioNTech received first U.S. FDA EUA of COVID-19 vaccine in children ages 5 through 11 years
On Oct. 29, 2021, Pfizer and BioNTech announced that the U.S. Food and Drug Administration (FDA) had authorized for emergency use the Pfizer-BioNTech COVID-19 Vaccine for children 5 through 11 years of age.
For this age group, the vaccine was to be administered in a two-dose regimen of 10-ᄉg doses given 21 days apart. The 10-ᄉg dose level was carefully selected based on safety, tolerability and immunogenicity data. This is the first COVID-19 vaccine authorized in the U.S. for individuals 5 through 11 years of age.
Tags:
Source: BioNTech
Credit:
