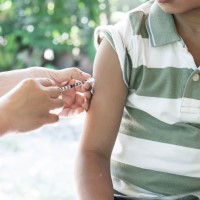
Pfizer and BioNTech granted U.S. EUA for booster dose of COVID-19 vaccine in children 5 through 11 years of age
On May 17, 2022, Pfizer and BioNTech announced the U.S. Food and Drug Administration expanded emergency use authorization to include a booster dose after completion of the primary series of the Pfizer-BioNTech COVID-19 Vaccine in children 5 through 11 years of age.
The booster dose is given at least five months after the second dose of the two-dose primary series and is the same 10-ᄉg dose of the Pfizer-BioNTech COVID-19 Vaccine. To date, more than 8 million 5- to 11-year-olds in the U.S. have completed a primary series.
Tags:
Source: Pfizer
Credit:
