OraSure received $13.6 million from BARDA to support InteliSwab COVID-19 rapid test 510(k) Clearance and CLIA Waiver
On Sept. 23, 2021, OraSure Technologies and the Biomedical Advanced Research Development Authority (BARDA) announced up to $13.6 million in funding for the Company to obtain 510(k) clearance and Clinical Laboratory Improvement Amendments (CLIA) waiver for OraSures InteliSwab COVID-19 rapid test from the Food and Drug Administration (FDA).
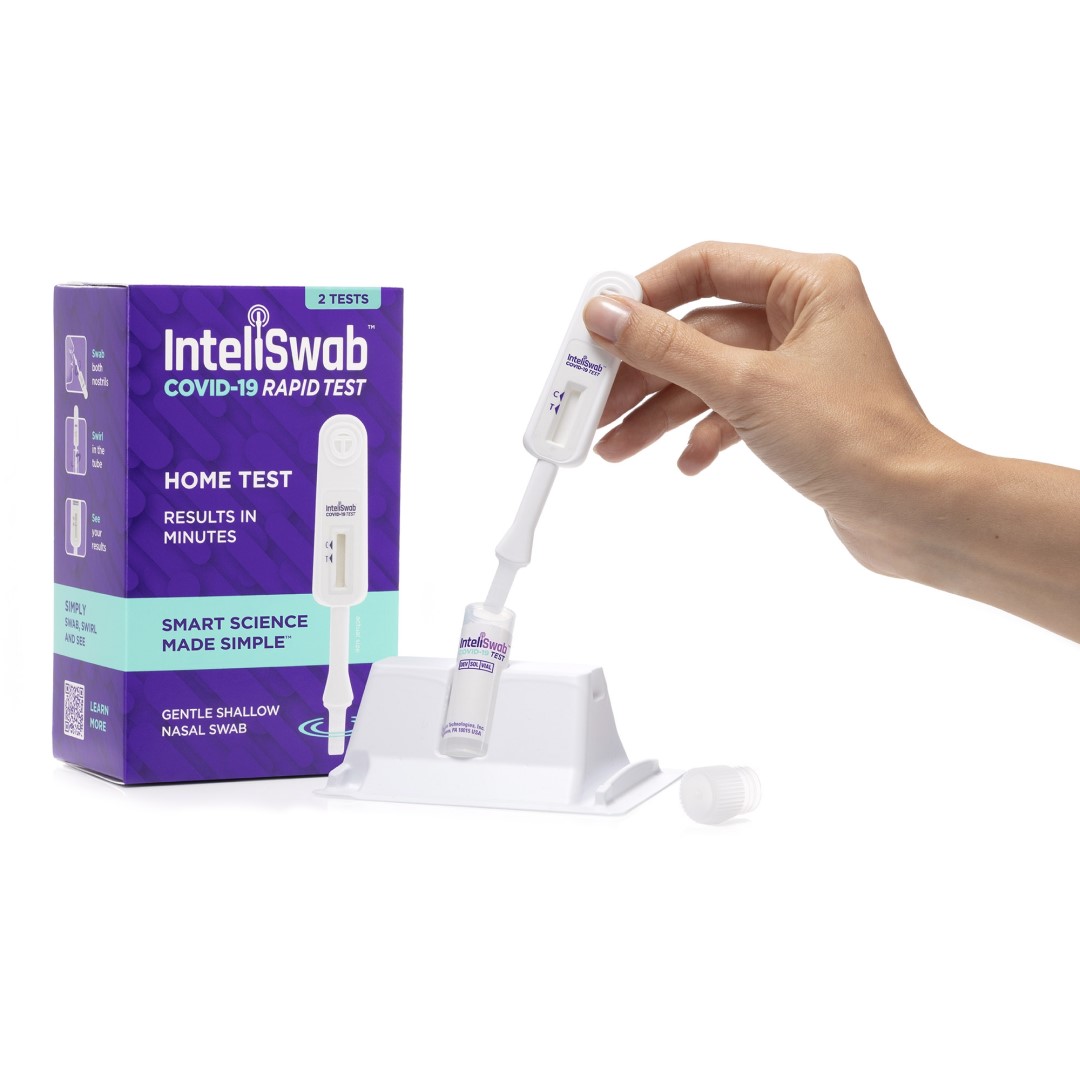
InteliSwab™ is a simple “swab, swirl, and see” test that uses an integrated swab to self-collect a sample from the lower nostrils. The result appears right on the test stick within 30 minutes, with no instruments, batteries, smartphone or laboratory analysis needed. It has three Emergency Use Authorizations from the Food and Drug Administration for professional point-of-care use, prescription (Rx) home use, and over-the-counter (OTC) use.
Tags:
Source: OraSure Technologies
Credit:
