NIH launched clinical trial of mRNA Nipah virus vaccine
On Jul. 11, 2022, the National Institute of Allergy and Infectious Diseases (NIAID) launched an early-stage clinical trial evaluating an investigational vaccine to prevent infection with Nipah virus. The experimental vaccine was manufactured by Moderna, and was developed in collaboration with NIAID’s Vaccine Research Center. It is based on a messenger RNA (mRNA) platformラa technology used in several approved COVID-19 vaccines. NIAID sponsored the Phase 1 clinical study, which is being conducted at the NIH Clinical Center in Bethesda, Maryland.
Nipah virus infection is a zoonotic disease, meaning that it is spread between animals and people. Fruit bats are the natural host for the virus. The first known Nipah outbreak occurred in 1998 in Malaysia and Singapore and resulted in 265 human cases and 105 deaths, and caused significant economic damage to the swine industry there. Since 1999, outbreaks have occurred annually in Asia, primarily in Bangladesh and India.
The virus can cause mild-to-severe disease rapidly progressing from respiratory infection symptoms to encephalitis (brain swelling) leading to coma or death. An estimated 40% to 75% of people infected with Nipah virus die. Although most cases are transmitted via animals, person-to-person transmission can occur. Currently, there is no licensed vaccine or treatment for Nipah virus infection.
Tags:
Source: National Institutes of Health
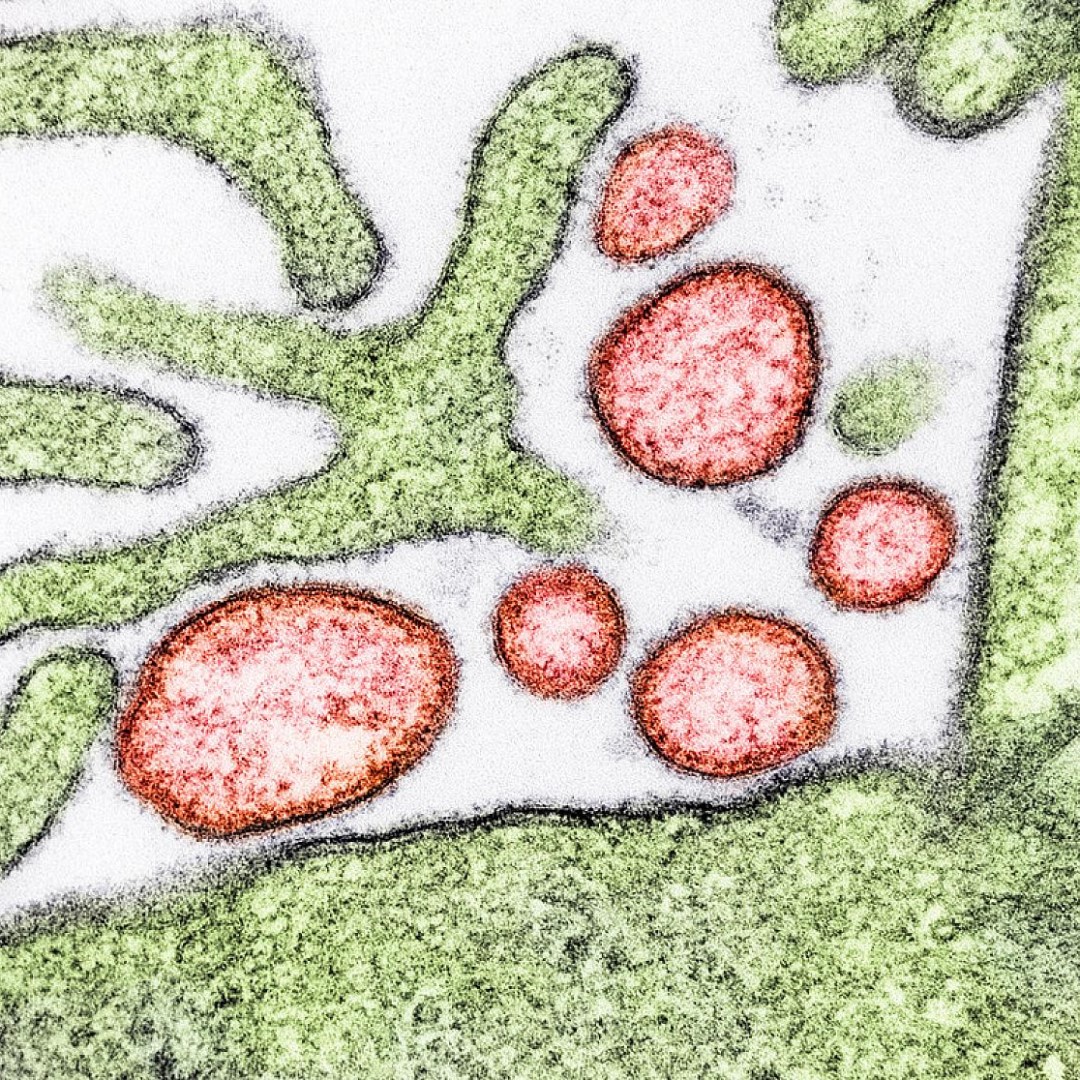
Credit: Photo: Colorized transmission electron micrograph of mature extracellular Nipah Virus particles (red) near the periphery of an infected VERO cell (green). Courtesy: National Institute of Allergy and Infectious Diseases.
