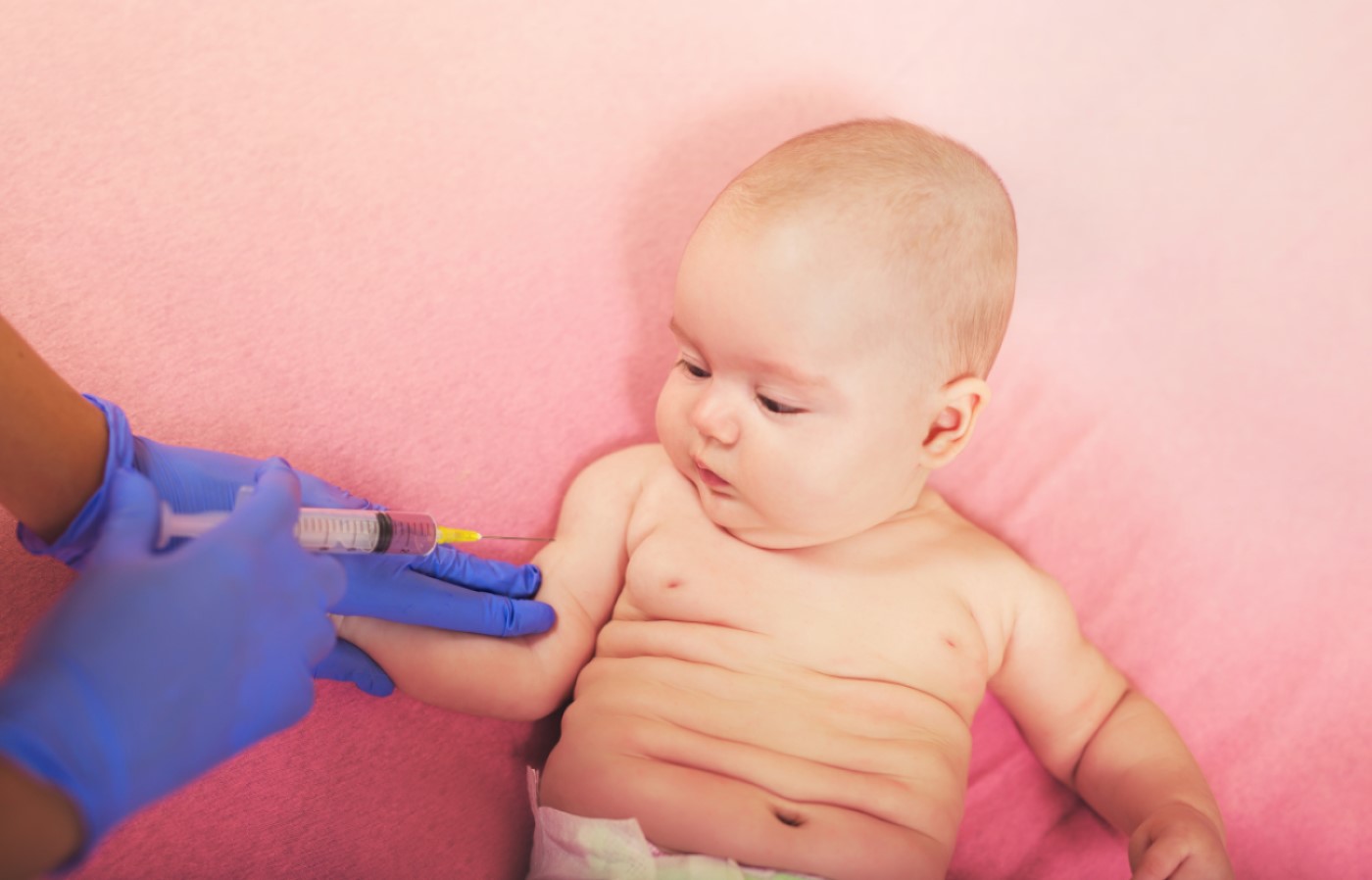
Moderna received FDA authorization for EUA of Its COVID-19 vaccine for children 6 months of age and older
On Jun. 17, 2022, Moderna announced that it had received emergency use authorization from the U.S. Food and Drug Administration for its COVID-19 vaccine (mRNA-1273) in young children ages 6 months through 5 years of age at a dose level of 25 ᄉg.
The company also received emergency use authorization for a 50 ᄉg two-dose regimen of mRNA-1273 for children ages 6 through 11 years old and a 100 ᄉg two-dose regimen for adolescents aged 12 through 17 years old. The two-dose regimens, with doses tailored for each age group given one month apart, are well-timed to initiate protection for the start of the school year, as children return to higher-risk classroom and daycare settings.
Tags:
Source: Moderna
Credit:
