GSK licensed tuberculosis vaccine candidate to the Bill & Melinda Gates Medical Research Institute for continued development
On Jan. 27, 2020, GSK announced that it had licensed its M72/AS01E[3] tuberculosis disease (TB) vaccine candidate to the Gates MRI, paving the way for continued development and potential use of the vaccine candidate in low-income countries with high TB burdens.
There was no approved vaccine capable of preventing pulmonary TB disease in adolescents and adults, who accounted for 89% of people who fell ill with TB in 2018. The live attenuated vaccine, Bacille Calmette-Guerin (BCG), has been in use for nearly a century, and while it is effective in preventing severe TB disease in infants and young children, it provides limited protection against pulmonary TB in adolescents and adults.
Tags:
Source: GlaxoSmithKline
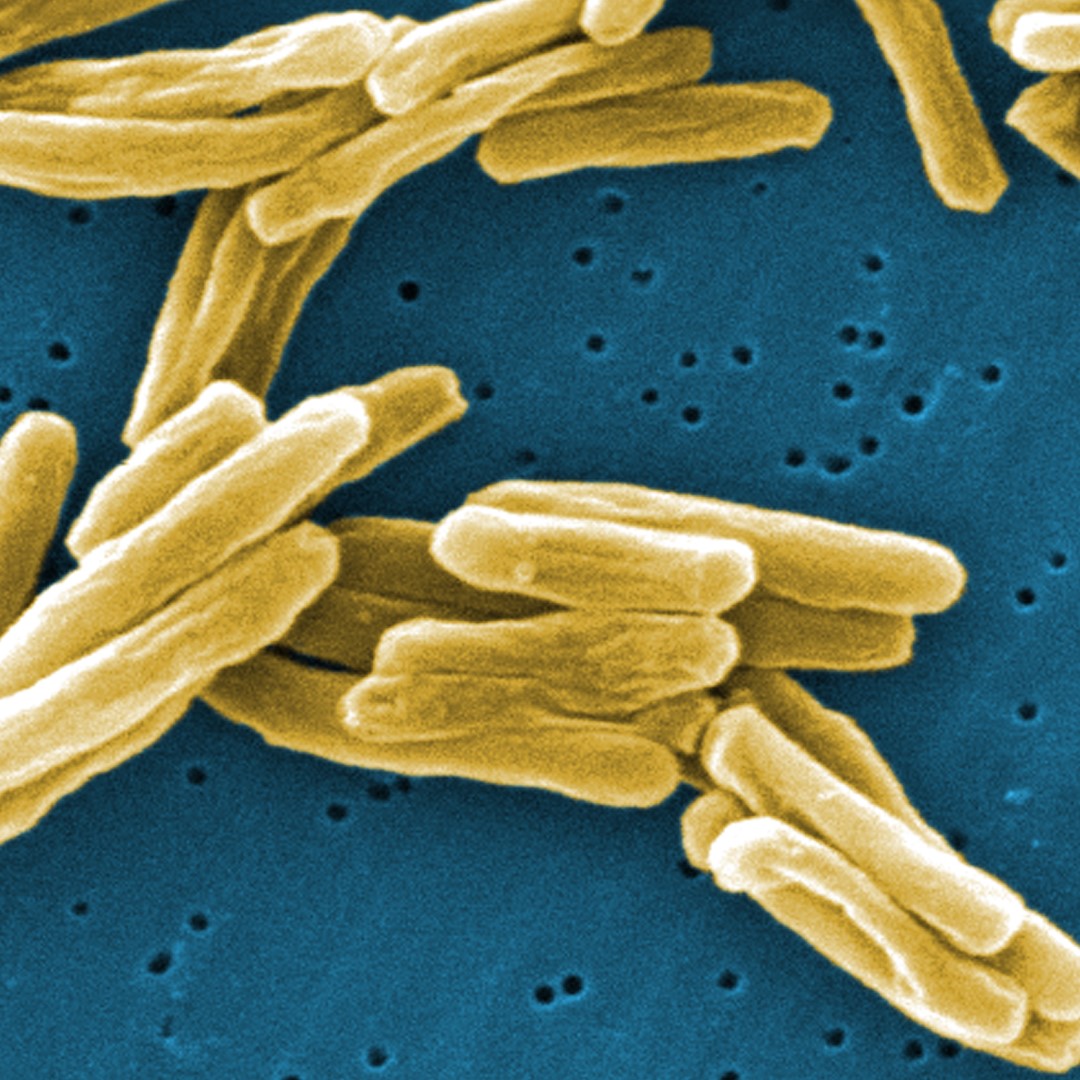
Credit: Photo: Mycobacterium tuberculosis bacteria, Centers for Disease Control and Prevention, courtesy Wikipedia.
