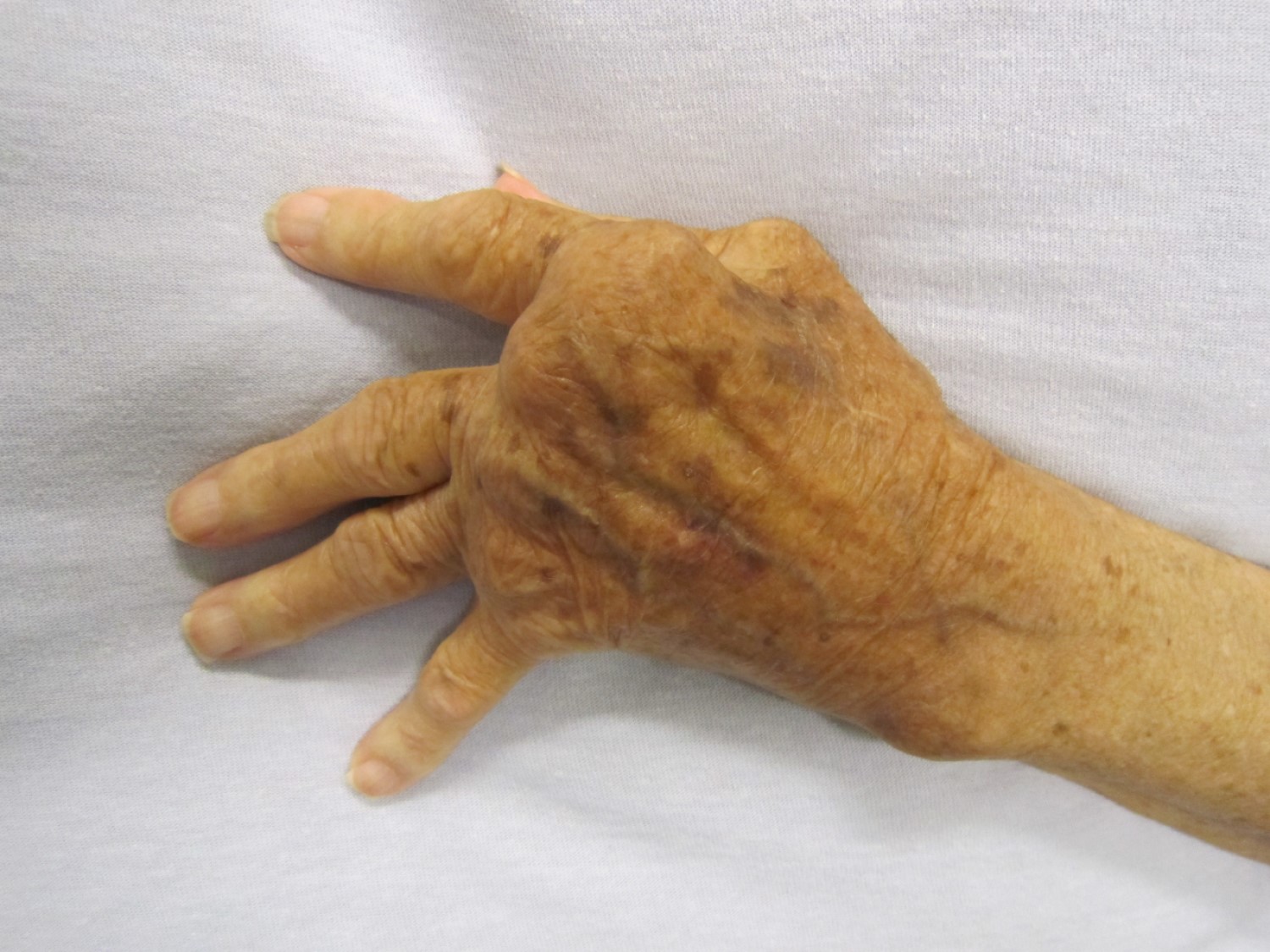
FDA Approves Tocilizumab for Treatment of Rheumatoid Arthritis
On Jan. 11, 2010, the U.S. Food and Drug Administration (FDA) approved Genentech’s tocilizumab (Actemra) for the treatment of patients with moderate to severe rheumatoid arthritis who have had inadequate clinical responses to treatment with TNF inhibitors.
Tocilizumab is a humanized monoclonal antibody directed against the receptor for IL-6, a cytokine increased in the blood and joints of RA patients and a contributor to many of the signs and symptoms of RA. The drug was administered as a monthly intravenous infusion dosed on weight.
Tags:
Source: Johns Hopkins Arthritis Center
Credit:
