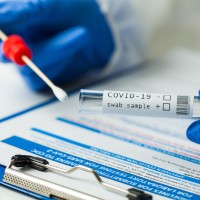
FDA authorized Cue Health’s COVID-19 test for at-mome and Over The Counter Use
On Mar. 5, 2021, Cue Health announced it had received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for over the counter sale of its fast, accurate, COVID-19 test, making it the nationメs first molecular diagnostic test available to consumers without a prescription.
The Cue COVID-19 Test for Home and Over The Counter (OTC) Use (Cue OTC Test) uses a lower nasal swab and delivers results in about 20 minutes to the userメs mobile smart device. The Cue OTC Test was authorized for use by symptomatic and asymptomatic individuals, adults and children ages 2 and older with adult assistance.
Tags:
Source: Cue Health
Credit:
