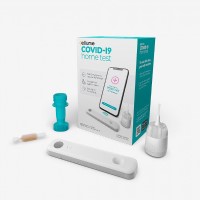
FDA authorized antigen test as first over-the-counter fully at-home diagnostic test for COVID-19
On Dec. 15, 2020, the FDA granted emergency use authorization (EUA) for the first over-the-counter (OTC) fully at-home diagnostic test for COVID-19.
The Ellume COVID-19 Home Test is a rapid, lateral flow antigen test, a type of test that runs a liquid sample along a surface with reactive molecules. The test detects fragments of proteins of the SARS-CoV-2 virus from a nasal swab sample from any individual 2 years of age or older.
Tags:
Source: U.S. Food and Drug Administration
Credit:
