FDA approved new sickle cell treatment based on clincal trial results from UTHSC’s Kenneth Ataga, MD
On Dec. 2, 2019, the U.S. Food and Drug Administration (FDA) announced it had approved the drug crizanlizumab based on the results of a clinical trial led by Kenneth Ataga, MD, of the Center for Sickle Cell Disease at the University of Tennessee Health Science Center.
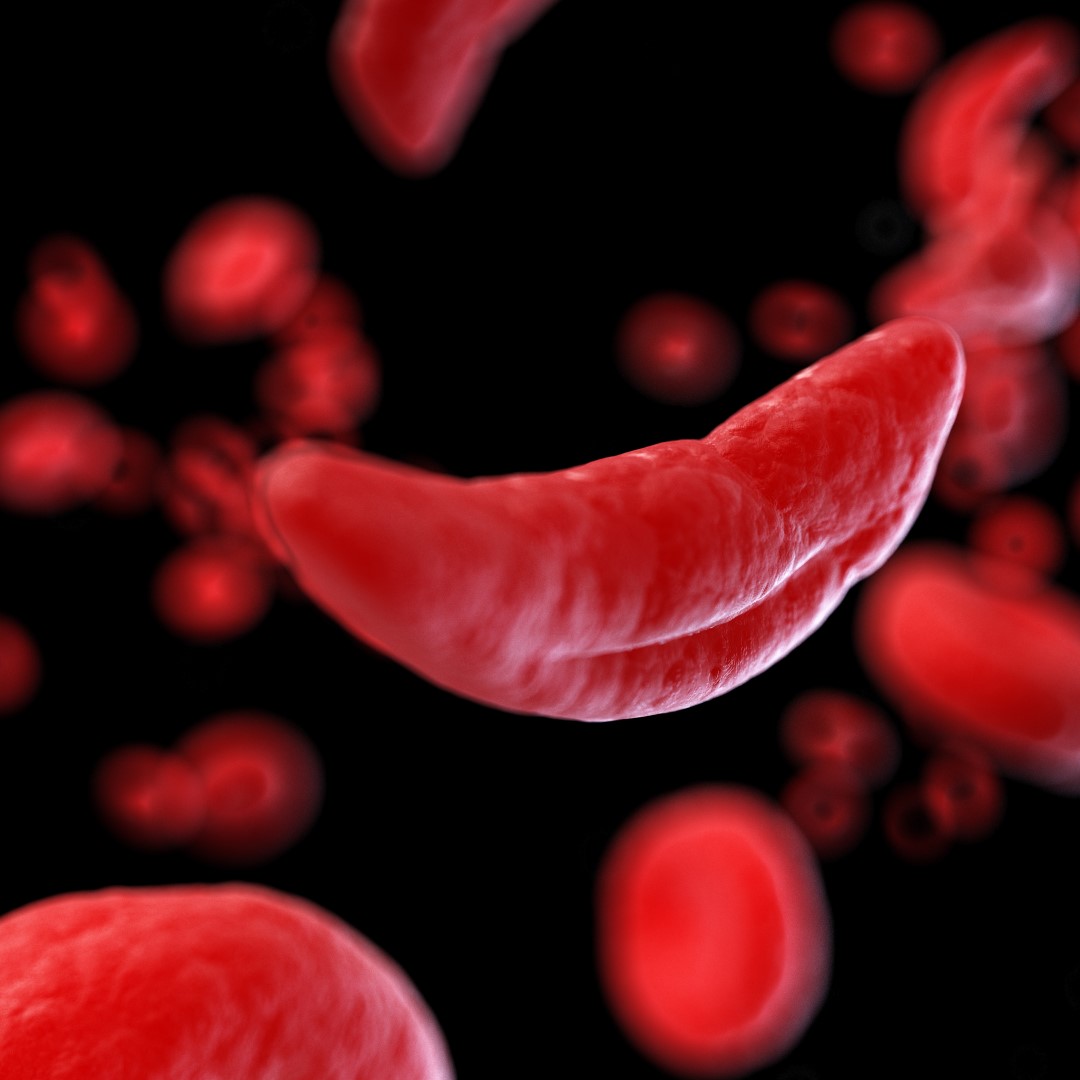
Sickle cell disease is an inherited blood disorder in which the red blood cells are abnormally shaped (in a crescent or “sickle” shape), which restricts the flow of blood and limits oxygen delivery to the body’s tissues, leading to severe pain and organ damage. According to the Centers for Disease Control and Prevention, sickle cell disease affects approximately 100,000 Americans.
Considered the clinical hallmark of the disease, sickle cell pain crises are unpredictable, severe events associated with life-threatening complications. They are triggered, in part, by multicellular interactions that form clusters of cells, which can block or reduce the blood flow to organs. Sickle cell pain crises are the main reason why individuals living with sickle cell disease go to the emergency room and are admitted to the hospital.
Phase II SUSTAIN was a 52-week randomized, placebo-controlled trial conducted as a double-blind study. A total of 198 patients with any genotype of sickle cell disease received either a 5mg/kg body weight or 2.5mg/kg body weight dose of crizanlizumab or a placebo. Results showed that the higher dose significantly lowered the median annual rate of VOCs by 45 percent (1.63 vs 2.98 compared to placebo).
Reductions in the frequency of VOCs were observed among patients regardless of sickle cell disease genotype and/or use of hydroxyurea, the only other FDA-approved treatment proven to reduce the frequency of painful episodes at the time of the trial. Results also showed that treatment with crizanlizumab increased the percentage of patients who did not experience any vaso-occlusive crises compared to the placebo.
Tags:
Source: The University of Tennessee Health Sciences Center News
Credit:
