FDA approved first gene therapies to treat patients with sickle cell disease
On Dec. 8, 2023, the U.S. Food and Drug Administration (FDA) approved two milestone treatments, Casgevy and Lyfgenia, representing the first cell-based gene therapies for the treatment of sickle cell disease (SCD) in patients 12 years and older.
Additionally, one of these therapies, Casgevy, was the first FDA-approved treatment to utilize a type of novel genome editing technology, signaling an innovative advancement in the field of gene therapy.
Casgevy, a cell-based gene therapy, is approved for the treatment of sickle cell disease in patients 12 years of age and older with recurrent vaso-occlusive crises. Casgevy is the first FDA-approved therapy utilizing CRISPR/Cas9, a type of genome editing technology. Patients’ hematopoietic (blood) stem cells are modified by genome editing using CRISPR/Cas9 technology. The FDA granted approval of Casgevy to Vertex Pharmaceuticals and approval of Lyfgenia to Bluebird Bio.
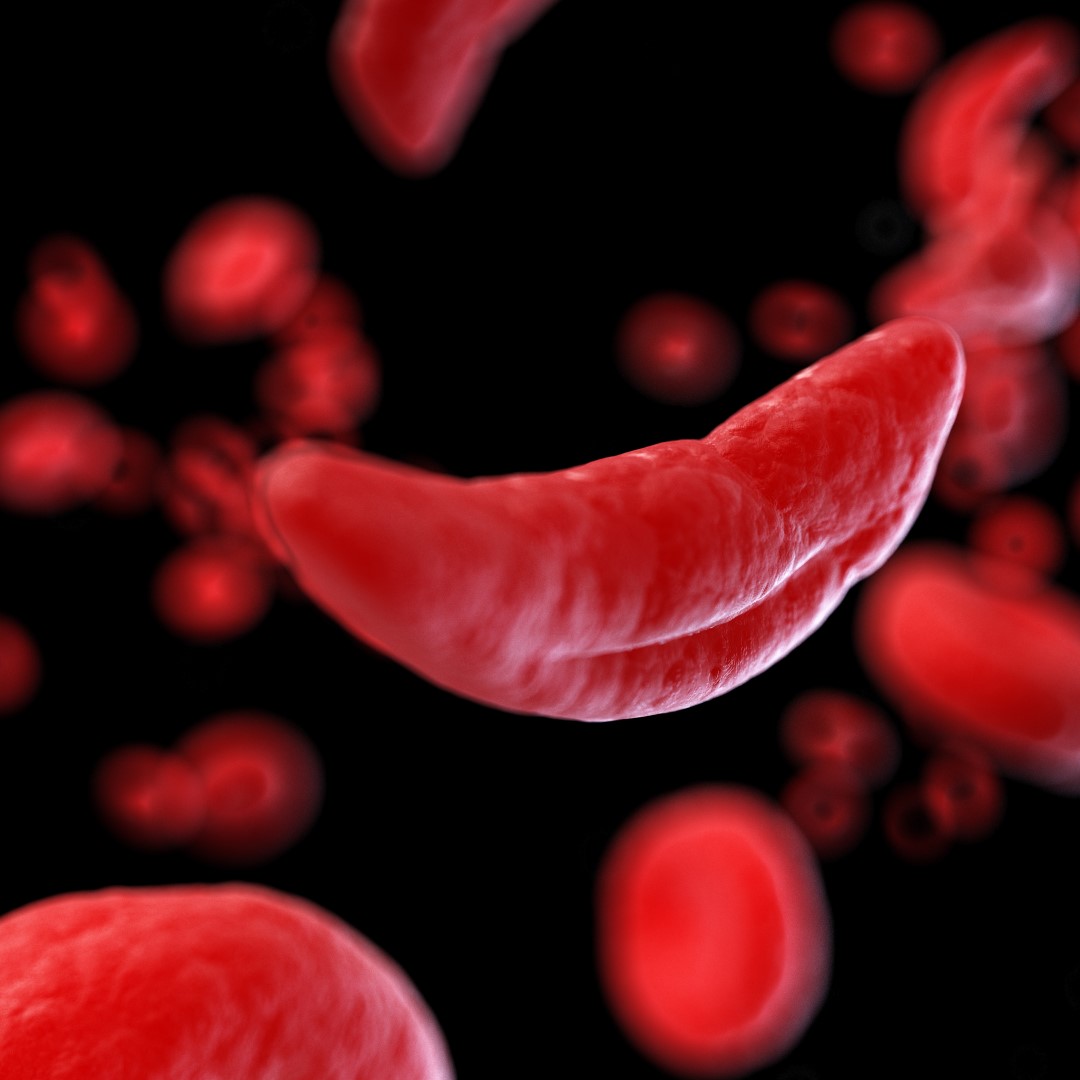
Sickle cell disease is a group of inherited blood disorders affecting approximately 100,000 people in the U.S. It is most common in African Americans and, while less prevalent, also affects Hispanic Americans. The primary problem in sickle cell disease is a mutation in hemoglobin, a protein found in red blood cells that delivers oxygen to the body’s tissues. This mutation causes red blood cells to develop a crescent or “sickle” shape.
These sickled red blood cells restrict the flow in blood vessels and limit oxygen delivery to the body’s tissues, leading to severe pain and organ damage called vaso-occlusive events (VOEs) or vaso-occlusive crises (VOCs). The recurrence of these events or crises can lead to life-threatening disabilities and/or early death.
Tags:
Source: U.S. Food and Drug Administration
Credit:
