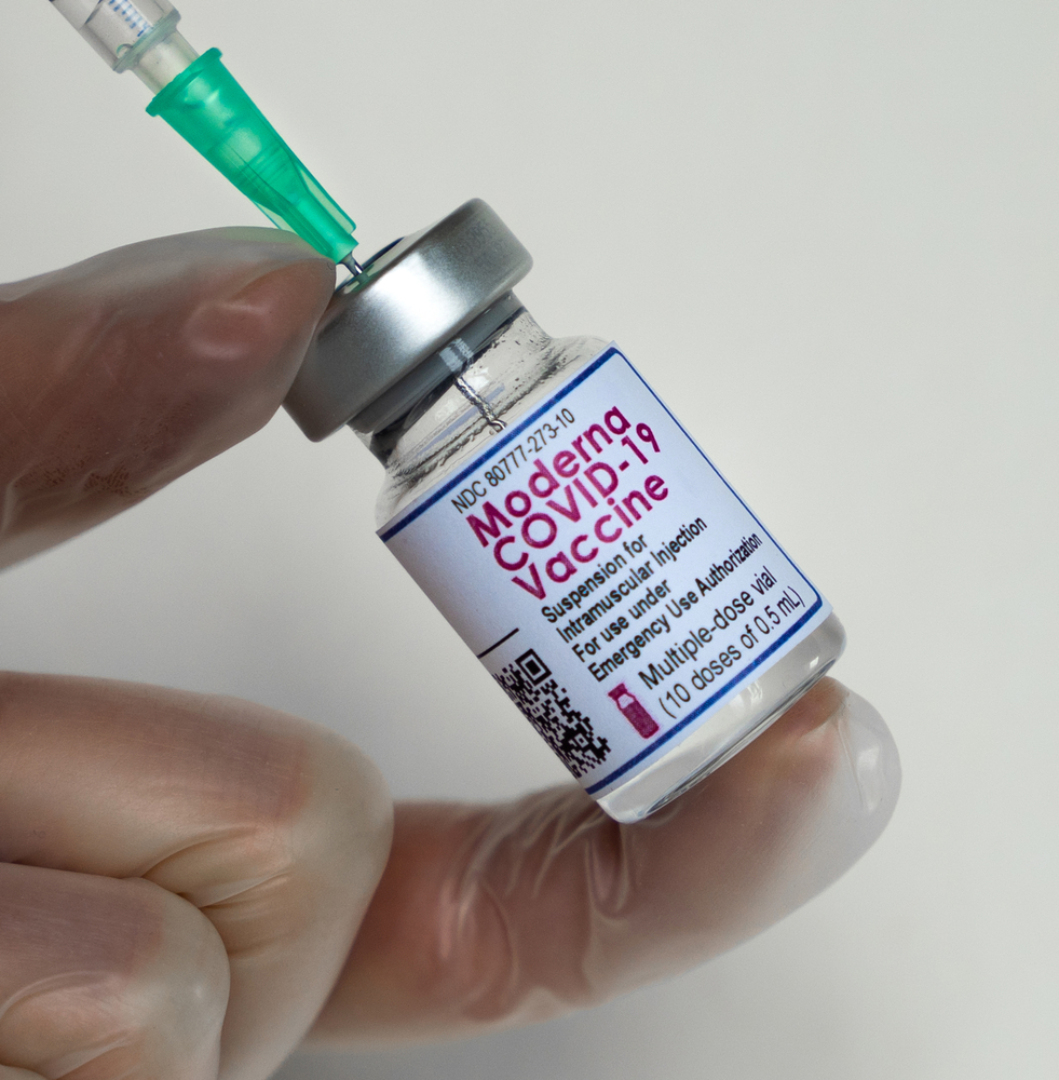
FDA announced FDA authorization of Moderna’s booster dose of COVID-19 vaccine in the U.S. for adults 18 years and older
On Nov. 19, 2021, the U.S. Food and Drug Administration (FDA) announced that it had extended the emergency use authorization of a booster dose of the Moderna COVID-19 vaccine at the 50 ᄉg dose level to all adults aged 18 and older. This booster can be used in all individuals 18 years and older who have completed a primary vaccination with any other authorized or approved COVID-19 vaccine.
Prior to this authorizations, a single booster dose of the Moderna and Pfizer-BioNTech COVID-19 vaccines was authorized for administration to individuals 65 years of age and older, individuals 18 through 64 years of age at high risk of severe COVID-19 and individuals 18 through 64 years of age with frequent institutional or occupational exposure to SARS-CoV-2.
Tags:
Source: U.S. Food and Drug Administration
Credit:
