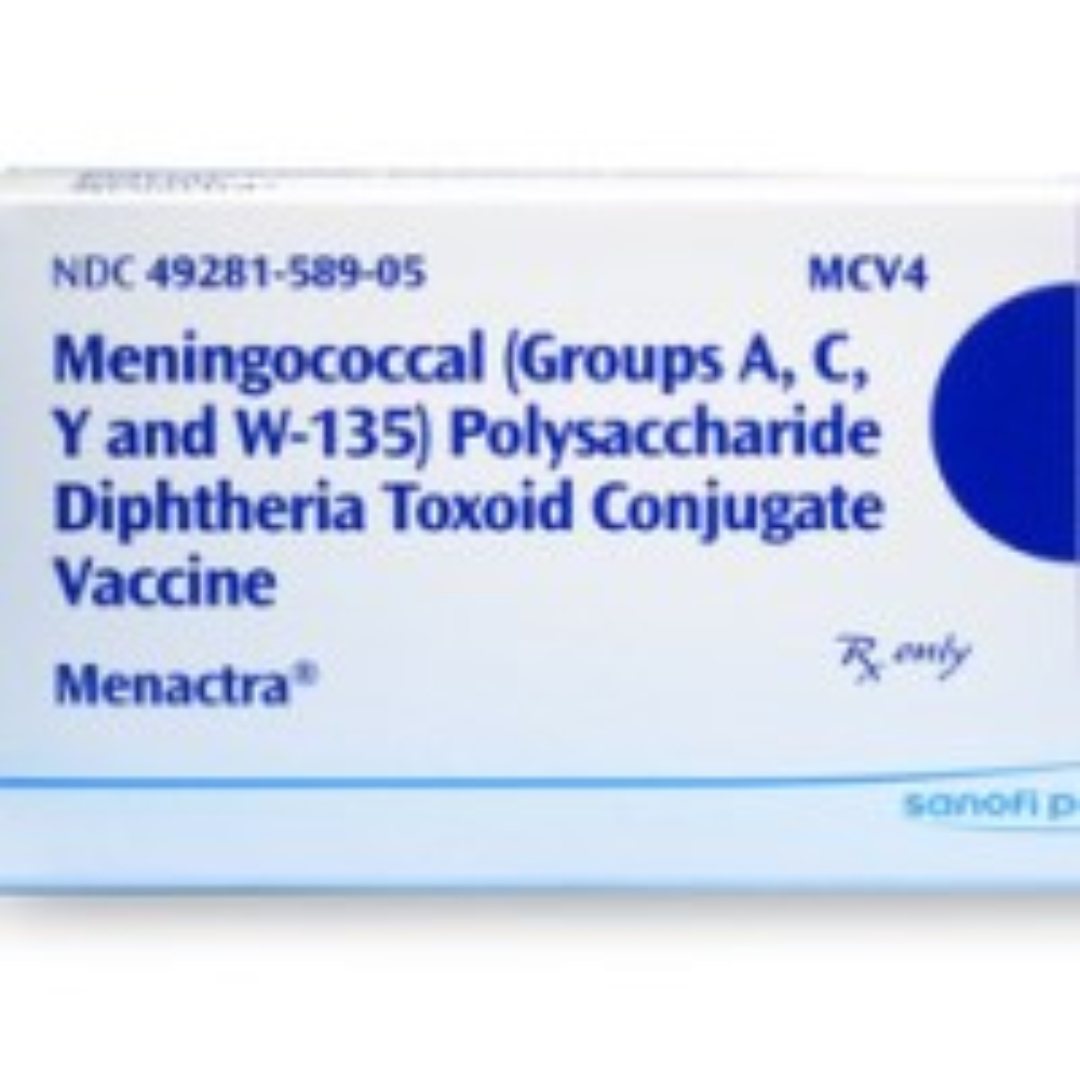
CDC published updated recommendation for meningococcal vaccination of at-risk children age 2-10 years in MMWR
On Oct. 17, 2007, the Food and Drug Administration (FDA) approved quadrivalent meningococcal conjugate vaccine (MCV4) (Menactra®, Sanofi Pasteur, Swiftwater, Pennsylvania) for use in children aged 2–10 years, in addition to its prior approval for use in persons aged 11–55 years.
Previous Advisory Committee on Immunization Practices (ACIP) recommendations called for routine vaccination with meningococcal polysaccharide vaccine (MPSV4) (Menomune®, Sanofi Pasteur) of children aged 2–10 years who are at increased risk for meningococcal disease.
Tags:
Source: U.S. Centers for Disease Control and Prevention
Credit:
