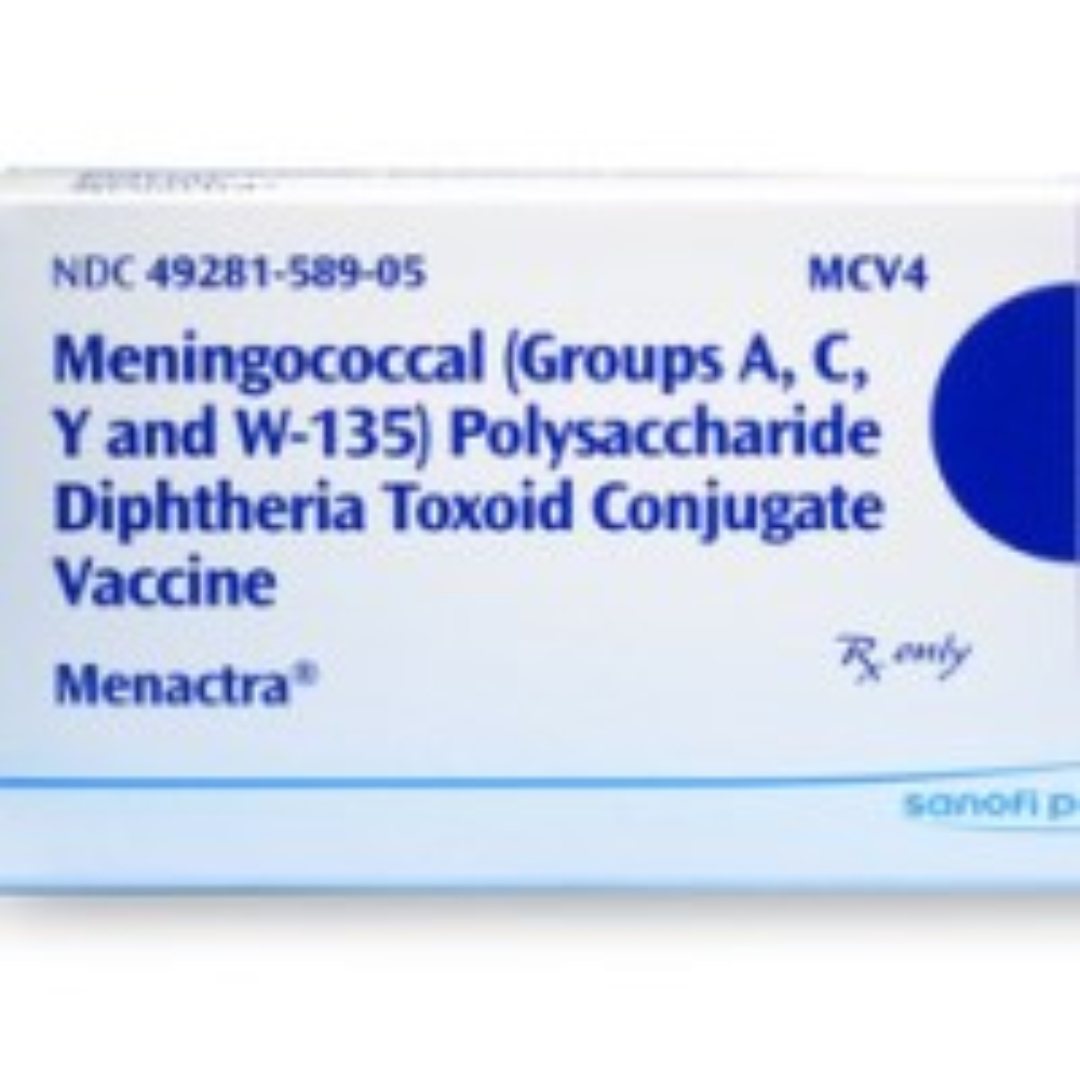
CDC published ACIP recommendations for use of meningococcal conjugate vaccines in HIV-infected persons
On Nov. 4, 2016, the U.S. Centers for Disease Control and Prevention’s (CDC) Immunization Practices Advisory Committee (ACIP) recommended routine use of meningococcal conjugate vaccine (serogroups A, C, W, and Y; including MenACWY-D [Menactra, Sanofi Pasteur] or MenACWY-CRM [Menveo, GlaxoSmithKline]) for persons aged ≥2 months with human immunodeficiency virus (HIV) infection.
Neisseria meningitidis, also known as meningococcus, is a Gram-negative bacterium that can cause meningitis and other forms of meningococcal disease such as meningococcemia, a life-threatening sepsis.
Tags:
Source: U.S. Centers for Disease Control and Prevention
Credit:
