75% of U.S. scientists who answered Nature poll consider leaving
On Mar. 27, 2025, a Nature survey of more than 1,200 scientists, three-quarters of the total respondents are…

On Mar. 27, 2025, a Nature survey of more than 1,200 scientists, three-quarters of the total respondents are…
On Mar. 26, 2025, research from the University of Minnesota College of Pharmacy, published in Communications Medicine, laid…

On Mar. 25, 2025, GSK announced that the US Food and Drug Administration (FDA) has approved Blujepa (gepotidacin)…

On Mar. 3, 2025, the Institute for Health Metrics and Evaluation reported that without urgent policy reform and…
On Feb. 27, 2025, a new government report adds to evidence that the HPV vaccine, once called dangerous…

On Feb. 24, 2025, breast cancer is the most common cancer in women worldwide, but survival chances vary…

On Feb. 20, 2025, researchers from the University of Washington and Seattle Children’s Research Institute announced findings from…

On Feb. 19, 2025, research from the Texas A&M College of Veterinary Medicine and Biomedical Sciences has discovered…

On Feb. 17, 2025, Stanford researchers have conducted the first large-scale screen of these inherited changes, called single…

On Feb. 12, 2025, a study led by researchers from the University of Washington announced that a new…

Jan. 24, 2025, the U.S. Food and Drug Administration (FDA) pulled draft guidance from its website requiring companies…

On Jan. 24, 2025, researchers from the Veterans Affairs Portland Health Care System in Oregon reported results from…

On Jan. 20, 2025, the White House announced the United States will exit the World Health Organization (WHO)….
On Jan. 20, 2025, a large international 68-country survey of 71,922 respondents found that in most countries, most…

On Jan. 10, 2025, the Institute for Health Metrics and Evaluation (IHME) reported that when it comes to…

On Jan. 3, 2025, the U.S. General Dr. Vivek Murthy released a new Surgeon General’s Advisory on Alcohol…

On Jan. 2, 2025, an international study led by researchers at Karolinska Institutet in Sweden shows that AI-based…

On Dec. 18, 2024, researchers from the UC San Francisco released a study showing that tires and degrading…

On Dec. 11, 2024, University of Pennsylvania Engineers announced they had made a critical breakthrough that bridges a…

On Dec. 5, 2024, researchers Dr Sonia Shah and Dr Clara Jiang from the University of Queensland’s Institute…

On Nov. 27, 2024, researchers at MUSC Hollings Cancer Center reported that cervical cancer deaths have plunged dramatically…

On Nov 20, 2024, researchers from the Wellcome Sanger Institute and collaborators have used cutting-edge genomic techniques to…

On Nov. 15, 2024, a team led by researchers from Sweden’s Karolinska Institutet reported study results that showed…

On Nov. 14, 2024, the March of Dimes released its 2024 Report Card, revealing the U.S. preterm birth…
On Nov 8, 2024, researchers from the Wellcome Sanger Institute celebrated a milestone of sequencing 50 Petabases of…

On Nov. 8, 2024, Anixa Biosciences announced updated positive data from the Phase 1 clinical trial of its…

On Oct. 24, 2024, researchers from Children’s Medical Center Research Institute at UT Southwestern reported that ancient viral remnants…
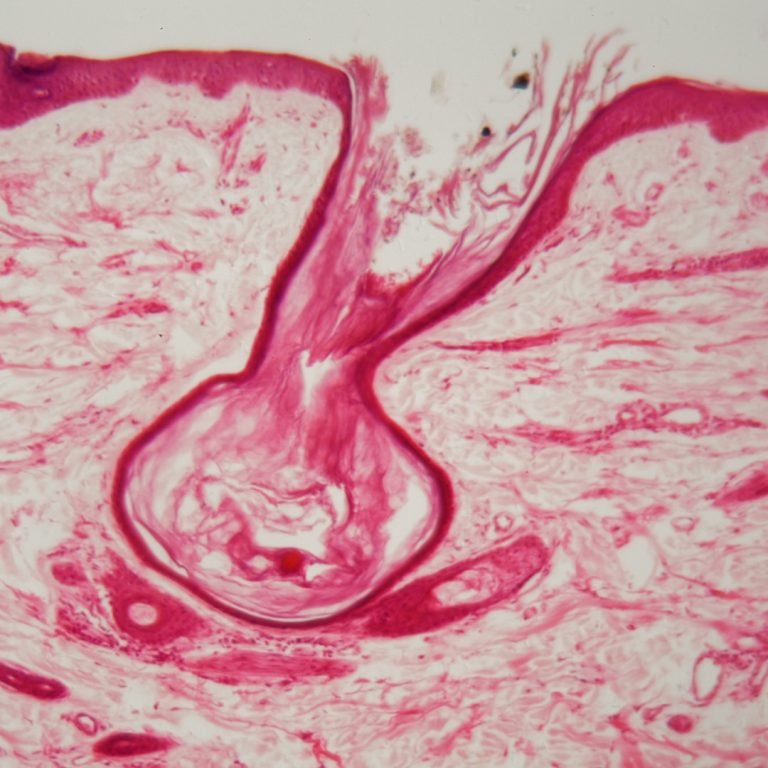
On Oct. 16, 2024, researchers from the Wellcome Sanger Institute, Newcastle University and their collaborators announced they had…

On Oct. 4, 2024, researchers from Oxford university announced they had been awarded funding from Cancer Research UK…

On Oct. 3, 2024, the National Health Service England (NHS) announced that Hundreds of babies have begun to…