CDC published updated dosing instructions for hepatitis A prophylaxis with immune globulin
On Sept. 15, 2017, the U.S. Centers for Disease Control and Prevention (CDC) published updated GamaSTAN S/D prescribing…
On Sept. 15, 2017, the U.S. Centers for Disease Control and Prevention (CDC) published updated GamaSTAN S/D prescribing…

On Sept. 6, 2017, PATH announced it has received a $120 million grant from the Bill & Melinda…

On Aug. 28, 2017, the American Academy of Pediatrics (AAP) issued policy stating that newborns should routinely receive…

On Aug. 25, 2017, the U.S. Centers for Disease Control and Prevention (CDC) published Immunization Practices Advisory Committee…

On Aug. 7, 2017, Seqirus announced that the accelerated development of cell-based manufacturing technology at its state-of-the-art manufacturing…
On Jul 6, 2017, a group led by virologist David Evans of the University of Alberta in Edmonton,…

On Jun. 30, 2017, the U.S. Centers for Disease Control and Prevention (CDC) and U.S. Food and Drug…

On Jun. 29, 2017, the U.S. Food and Drug Administration (FDA) unveiled a strategic plan to completely eliminate…

On Jun. 15, 2017, the U.S. Department of Health and Human Services (HHS) published the nations’s updated Pandemic…
On May 19, 2017, the U.S. Centers for Disease Control and Prevention (CDC) published Immunization Practices Advisory Committee…

On Apr. 21, 2017, the U.S. Centers for Disease Control and Prevention (CDC) published updated guidelines for use…

On Feb. 10, 2017, the Advisory Committee on Immunization Practices (ACIP) approved the Recommended Immunization Schedule for Children…

On Feb. 10, 2017, the U.S. Centers for Disease Control and Prevention (CDC) published the 2017 U.S. recommended…
On Dec. 14, 2016, Medigen Vaccine Biologics (MVC) amended an existing agreement with the National Institutes of Health…
On Nov. 18, 2016, the U.S. Food and Drug Administration (FDA) approved extending the age range for use…
On Nov. 4, 2016, the U.S. Centers for Disease Control and Prevention’s (CDC) Immunization Practices Advisory Committee (ACIP)…

On Oct. 27, 2016, Roswell Park Cancer Institute announced it had received authorization from the U.S. Food and…
On Sept. 27, 2016, the Region of the Americas was the first in the world to have eliminated…

On Sept. 13, 2016, Arbutus Biopharma announced that Dr. Michael J. Sofia, Arbutus’ Chief Scientific Officer, had been…

On Aug. 29, 2016, Seqirus announced that the U.S. Food and Drug Administration (FDA) had approved AFLURIA® QUADRIVALENT…

On Aug. 29, 2016, the American Academy of Pediatrics (AAP) released new policy statement that urged states to…

On Aug. 26, 2016, the U.S. Centers for Disease Control and Prevention (CDC) published 2016-17 influenza vaccination recommendations….
On Aug. 15, 2016, Siteman Cancer Center received a $10.4 million five-year grant to Washington University researchers and…

On Jun. 22, 2016, the U.S. Centers for Disease Control and Prevention’s (CDC) Immunization Practices Advisory Committee (ACIP)…
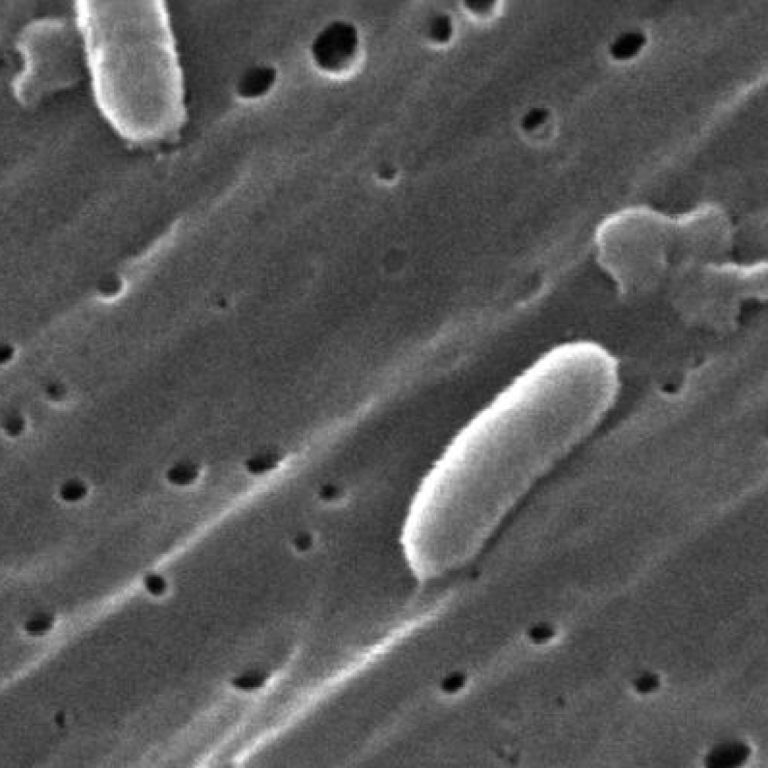
On Jun. 10, 2016, the U.S. Food and Drug Administration (FDA) approved Vaxchora, a vaccine for the prevention…

On Jun. 7, 2016, IBio and the Texas A&M Institute of Infectious Animal Diseases (TAMUS) announced they had…

On Sept. 5, 2017, business incubator TEC Edmonton announced a strategic partnership with Merck Canada to provide new…

On Apr. 29, 2016, GlaxoSmithKline Biologicals in Research Triangle Park, North Carolina, received U.S. Food and Drug Administration…

On Mar. 14, 2016, Inovio Pharmaceuticals announced an agreement to acquire all of Bioject Medical Technologies’ assets including…
On Feb. 17, 2016, MD Anderson Cancer Center was awarded $14 million from the Cancer Prevention and Research…