Heat Biologics collaborated with University of Miami to develop COVID-19 Coronavirus vaccine
On Mar. 18, 2020, Immunology researchers at the University of Miami Miller School of Medicine announced a collaboration…
On Mar. 18, 2020, Immunology researchers at the University of Miami Miller School of Medicine announced a collaboration…

On Mar. 17, 2020, Pfizer and BioNTech SE announced the companies have agreed to a letter of intent…
On Mar. 17, 2020, CanSino Biologics announced that its recombinant novel Coronavirus Vaccine (Adenovirus Type 5 Vector) candidate…

On Mar. 17, 2020, Innovation Pharma announced details on the Material Transfer Agreement signed with a leading public…

On Mar. 16, 2020, the National Institutes of Health (NIH announced that a Phase 1 clinical trial evaluating…

On Mar. 16, 2020, Moderna announced that the first participant had been dosed in the Phase 1 study…
On Mar. 15, 2020, Fosun Pharma and BioNTech announced a strategic development and commercialization collaboration to advance BioNTech’s…

On Mar. 13, 2020, Johnson & Johnson announced that its Janssen Pharmaceutical Companies have entered a collaboration with…

On Mar. 12, 2020, Predictive Oncology announced it will launch a new AI platform for vaccine and drug…

On Mar. 11, 2020, Vir Biotech announced a research collaboration agreement with the NIH to advance characterization and…

On Mar. 10, 2020, CaroGen and scientists from Yale announced they had begun work on a vaccine targeting…

On Mar. 10, 2020, the Bill & Melinda Gates Foundation, Wellcome, and Mastercard committed up to $125 million…
On Mar. 3, 2020, Moderna announced that enrollment was complete for all three dose cohorts of the Phase…

On Mar. 3, 2020, Heat Biologics announced the Company has formally launched a program within its wholly-owned subsidiary,…
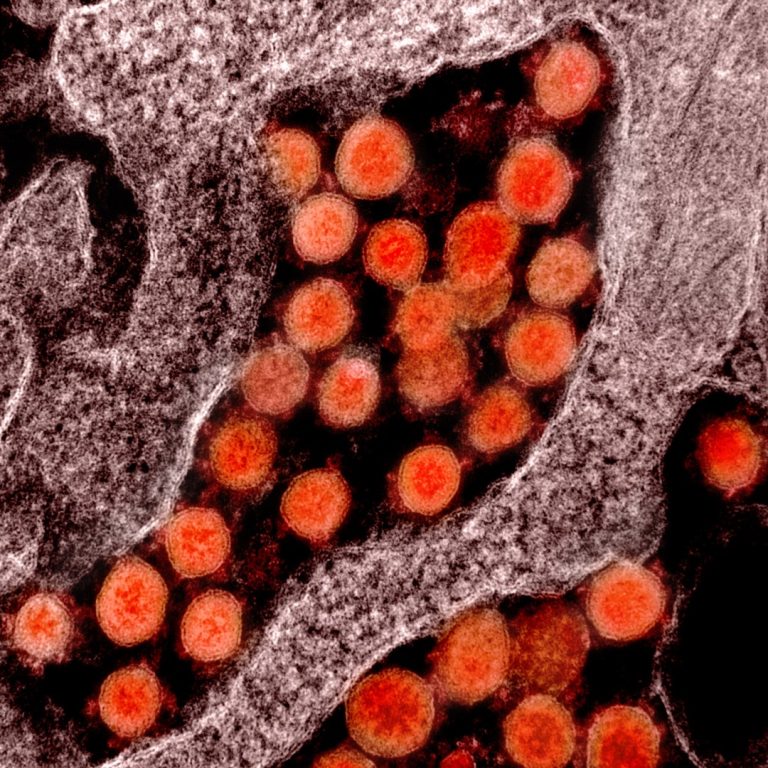
On Mar. 2, 2020, Boston’s top infectious disease researchers, including scientists from Boston University’s National Emerging Infectious Diseases…

On Mar. 1, 2020, the United Nations Humanitarian Chief Mark Lowcock released US$15 million from the Central Emergency…

On Feb. 28, 2020, commentary appeared in The New England Journal of Medicine that discussed the emergence and…

On Feb. 28, 2020, Altimmune announced the advancement of a novel single-dose, intranasal vaccine using Altimmune’s proprietary technology…

On Feb. 26, 2020, Novavax announced progress in its efforts to develop a novel vaccine to protect against…
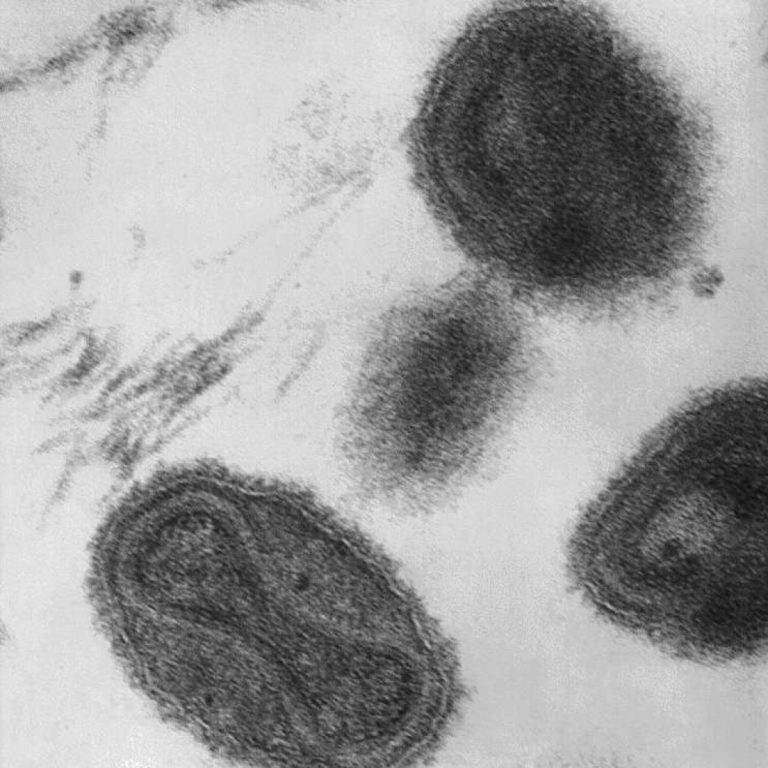
On Feb. 26, 2020, Tonix Pharmaceuticals announced a strategic collaboration with Southern Research to support the development of…
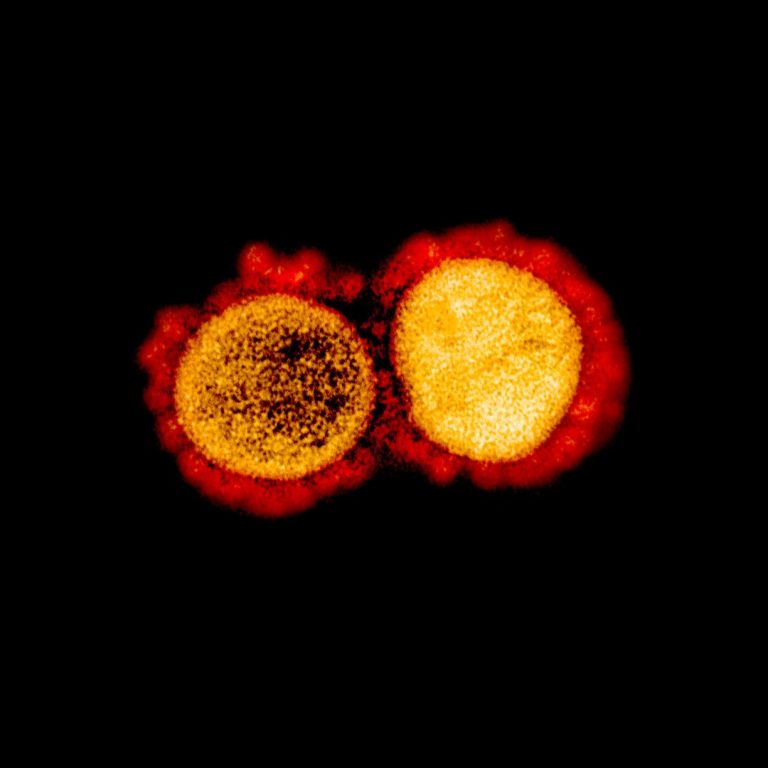
On Feb. 24, 2020, Scientists at Texas Biomed began work on the novel coronavirus known as SARS-CoV-2. A…

On Feb. 24, 2020, Seqirus announced that the U.S. Food and Drug Administration (FDA) had approved the first…

On Feb. 24, 2020, researchers at National Institute of Allergy and Infectious Disease’s Rocky Mountain Laboratories in Hamilton,…

On Feb. 24, 2020, Clover Biopharmaceuticals announced a research collaboration with GSK for its protein-based coronavirus vaccine candidate…

On Feb. 24, 2020, Moderna announced it had released the first batch of mRNA-1273, the Company’s vaccine against…
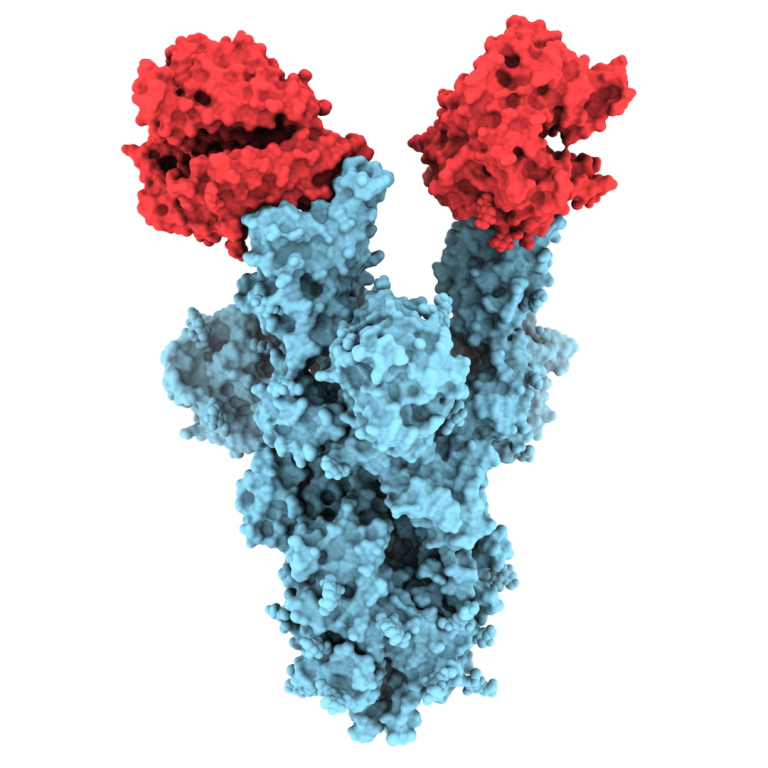
On Feb. 24, 2020, researchers reported that the spikes crowning the new coronavirus that causes COVID-19 atypical pneumonia…

On Feb. 19, 2020, NIAID scientists working with investigators from the University of Texas at Austin identified the…

On Feb. 18, 2020, Sanofi announced it had leveraged previous development work for a SARS vaccine which may…

On Feb. 16, 2020, Medigen Vaccine Biologics (MVB) and the National Institutes of Health (NIH) announced they had…

On Feb. 4, 2020, the Fred Hutchinson Cancer Research Center announced that a promising HIV vaccine trial was…