INOVIO COVID-19 DNA vaccine INO-4800 demonstrated robust neutralizing antibody and T cell immune responses in preclinical models
On May 20, 2020, INOVIO announced the publication of the preclinical study data for IN0-4800, its COVID-19 DNA…

On May 20, 2020, INOVIO announced the publication of the preclinical study data for IN0-4800, its COVID-19 DNA…

On May 20, 2020, Bharat Biotech and Thomas Jefferson University of Philadelphia announced an exclusive deal to develop…
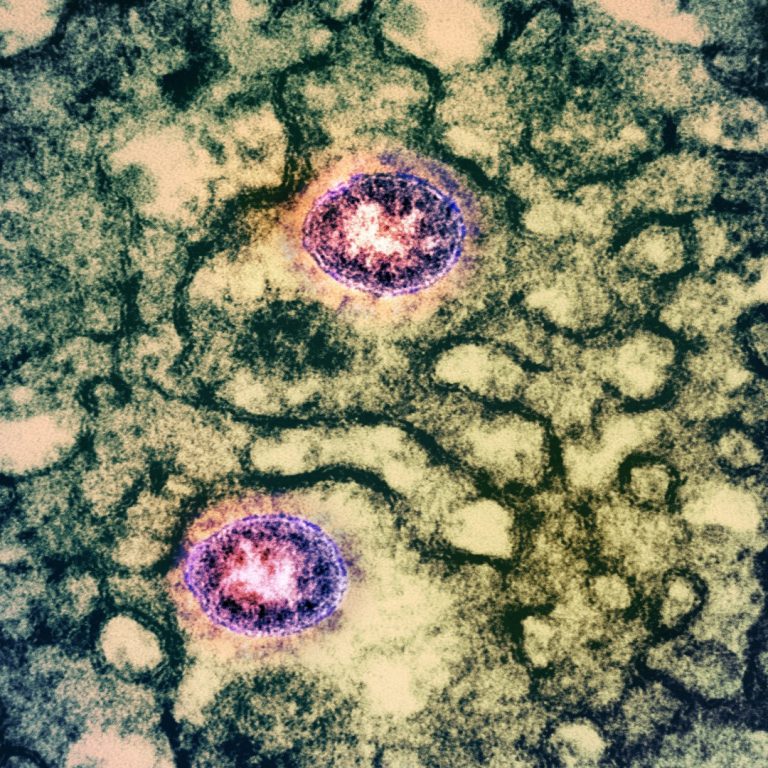
On May 19, 2020, in a study published in Cell Reports, researchers at the University of Hong Kong…

On May 19, 2020, Oragenics announced, that through its wholly-owned subsidiary, Noachis Terra, it has entered into an…

On May 18, 2020, Moderna announced positive interim clinical data of mRNA-1273, its vaccine candidate against novel coronavirus…

On May 16, 2020, a single dose of ChAdOx1 nCoV-19, an investigational vaccine against SARS-CoV-2, has protected six…

On May 14, 2020, scientists at La Jolla Institute for Immunology (LJI) announced they had resolved the uncertainty…
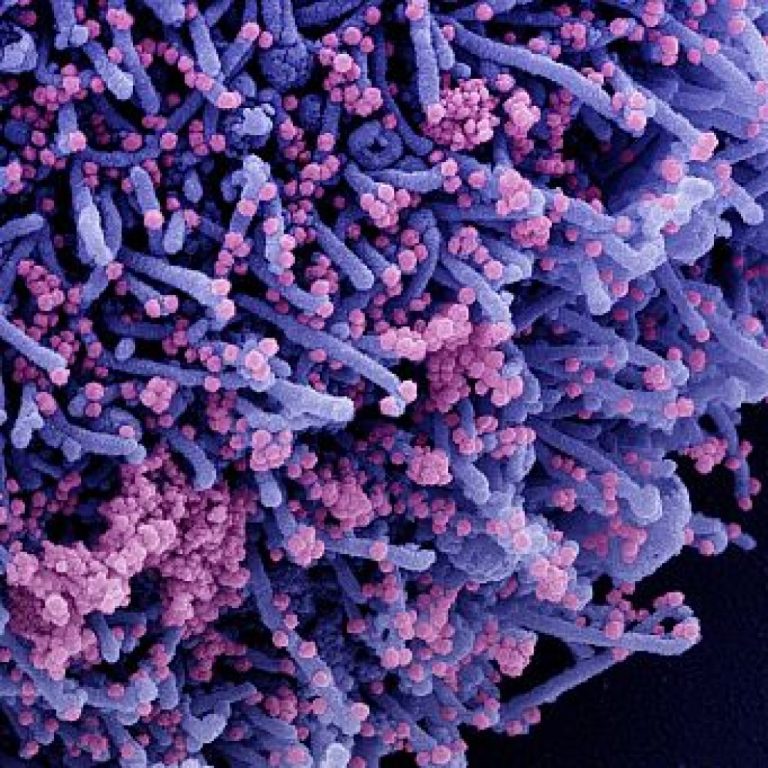
On May 13, 2020, University of Alberta (UA) researchers launched a joint effort with U.S.-based Tonix Pharmaceuticals to…

On May 12, 2020, the National Research Council of Canada (NRC) announced a collaboration with CanSino Biologics to…

On May 12, 2020, the National Research Council of Canada (NRC) and the University of Saskatchewan’s (USask) Vaccine…

On May 12, 2020, the Jackson Laboratory (JAX), in partnership with the State of Connecticut, announced that it…

On May 12, 2020, Moderna announced the U.S. Food and Drug Administration (FDA had granted Fast Track designation…

On May 11, 2020, a harmonized and collaborative approach to the clinical testing, scale-up and distribution of candidate…

On May 11, 2020, Thermo Fisher Scientific announced it is expanding capacity for viral vector development and manufacturing…

On May 11, 2020, Novavax announced that the Coalition for Epidemic Preparedness Innovations (CEPI) had committed to investing…

On May 11, 2020, in a perspective published by the journal Science, Dr. Larry Corey from the Fred…
On May 7, 2020, Entos Pharmaceuticals announced a partnership with PrecisionNanoSystems (PNI) to produce clinica lgrade vaccines and…

On May 7, 2020, Tonix Pharmaceuticals and the University of Alberta announced a research collaboration and exclusive licensing…

On May 6, 2020, Sinovac Biotech announced publication of the preclinical study on animals for its vaccine candidate…

On May 6, 2020, Predictive Oncology announced the acquisition of Soluble Therapeutics and partnership and licensing of a…

On May 5, 2020, Pfizer and BioNTech announced the first participants have been dosed in the U.S. in…

On May 5, 2020, Medigen Vaccine Biologics (MVB) announced a global commercial license agreement with National Institutes of…

On May 6, 2020, Lineage Cell Therapeutics announced that it has applied for grant funding from the California…

On May 5, 2020, Tyson Foods announced resumption of limited production at its Waterloo, Iowa facility May 7….

On May 5, 2020, Massachusetts Eye and Ear and Massachusetts General Hospital (MGH), members of Mass General Brigham,…

On May 4, 2020, Arcturus Therapeutics and Catalent announced a partnership to support the expected manufacture of Arcturusメ…

On May 1, 2020, Moderna and Lonza announced a 10-year strategic collaboration agreement to enable larger scale manufacture…

On Apr. 30, 2020, AstraZeneca and the University of Oxford announced an agreement for the global development and…

On Apr. 30, 2020, University of Oxford announced an agreement with the UK-based global biopharmaceutical company AstraZeneca for…

On Apr. 30, 2020, Valneva and Pfizer announced a collaboration to develop and commercialize Valneva’s Lyme disease vaccine…