Mateon selected by IBM Watson Health for use of IBM Clinical Development Solution to support clinical COVID-19 vaccine candidates
On Jun. 24, 2020, Oncotelic, a wholly owned subsidiary of Mateon Therapeutics, announced that IBM has granted access…

On Jun. 24, 2020, Oncotelic, a wholly owned subsidiary of Mateon Therapeutics, announced that IBM has granted access…

On Jun. 24, 2020, a team of scientists at Texas A&M University announced it is working with iBio…

On Jun. 24, 2020, Symbiosis Pharmaceutical Services, an India-based specialist provider of pharmaceutical and vaccine development services, announced…

On Jun. 23, 2020, the NIH announced it had tested a モuniversal mosquito vaccineヤ known as AGS-v. It…

On Jun. 23, 2020, scientists at Scripps Research reported that some common strains of influenza have the potential…

On Jun. 23, 2020, Sanofi and Translate Bio announced they had agreed to expand their existing 2018 collaboration…

On Jun. 23, 2020, INOVIO announced it had received $71 million funding from the U.S. Department of Defense…
On Jun. 22, 2020, Intravacc announced promising results of a preclinical study with its experimental whooping cough vaccine…

On Jun. 22, 2020, Pfizer announced the initiation of four phase 3 clinical trials within its current pipeline…

On Jun. 22, 2020, Taconic Biosciences announced an exclusive agreement with the University of Texas Medical Branch at…

On Jun. 19, 2020, GSK announced that a scientific collaboration with Clover Pharmaceuticals to develop an adjuvanted COVID-19…

On Jun. 19, 2020, Dynavax Technologies announced the first participants were dosed in the Phase 1 clinical trial…

On Jun. 18, 2020, Tonix Pharmaceuticals announced an expansion of its strategic collaboration with Southern Research to include…

On Jun. 17, 2020, Bvlgari, the Italian luxury brand, announced plans to support research into COVID-19 and vaccine…

On Jun. 16, 2020, lower-income countries across the world have access life-saving pneumococcal conjugate vaccines, which protect against…

On Jun. 15, 2020, Catalent announced that it will provide vial filling and packaging capacity to AstraZeneca at…

On Jun. 15, 2020, Batavia Biosciences and Valneva announced a collaboration agreement to accelerate market-access of a low-cost…

On Jun. 13, 2020, AstraZeneca announced an agreement with Europeメs Inclusive Vaccines Alliance (IVA), spearheaded by Germany, France,…

On Jun. 13, 2020, Sinovac Biotech announced positive preliminary results of phase I/II clinical trial for the Companyメs…

On Jun. 11, 2020, Sinovac Biotech announced the signing of a clinical development collaboration agreement to advance the…

On Jun. 11, 2020, Emergent BioSolutions announced deployment of its molecule-to-market contract development and manufacturing (CDMO) services to…

On Jun. 11, 2020, Moderna announced progress on late-stage development of mRNA-1273, the Company’s mRNA vaccine candidate against…

On Jun. 10, 2020, Johnson & Johnson announced that through its Janssen Pharmaceutical subsidiary it has accelerated the…

On Jun. 10, 2020, Catalent announced that it had signed an agreement with Spicona to develop a virus-like…
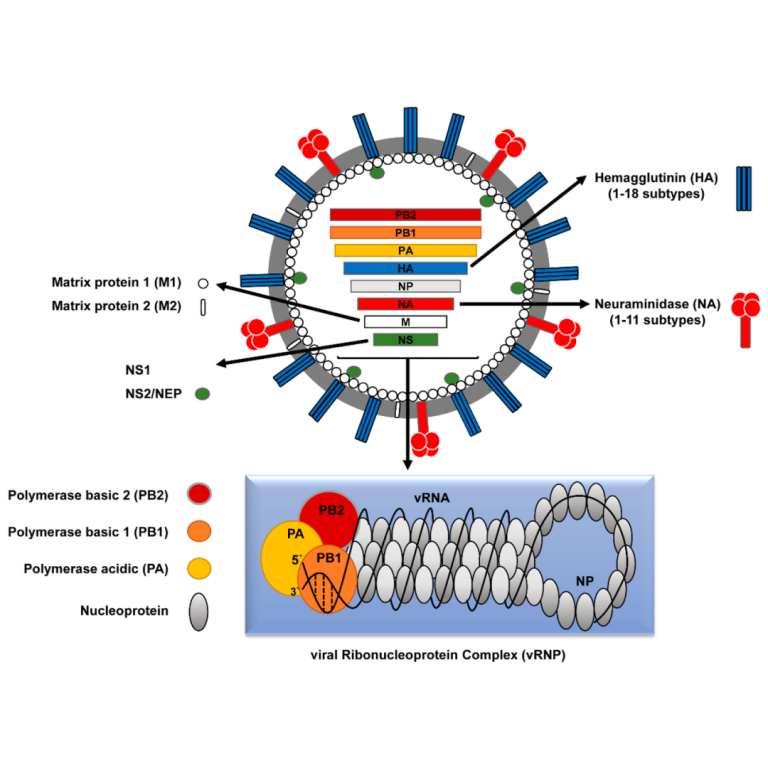
On Jun. 8, 2020, Intravacc and Versatope announced that they have signed a research service agreement to further…

On Jun. 5, 2020, the Alberta Precision Laboratories (APL) COVID-19 Biorepository was announced to ensure researchers have the…

On Jun. 5, 2020, the UK thelped secure $7.4 billion in funding to support global vaccine supply and…

On Jun. 5, 2020, CEPI, the Coalition for Epidemic Preparedness Innovations, CSL and The University of Queensland (UQ)…

On Jun. 4, 2020, world leaders pledged an additional US$ 8.8 billion for Gavi, the Vaccine Alliance, far…

On Jun. 4, 2020, vaccine manufacturers MSD, GSK, Innovax, Serum Institute of India and Walvax pledged to ramp…