Experimental coronavirus vaccine reported safe and produces immune response
On Jul. 21, 2020, researchers from the National Institutes of Health (NIH) announced that early results from the…
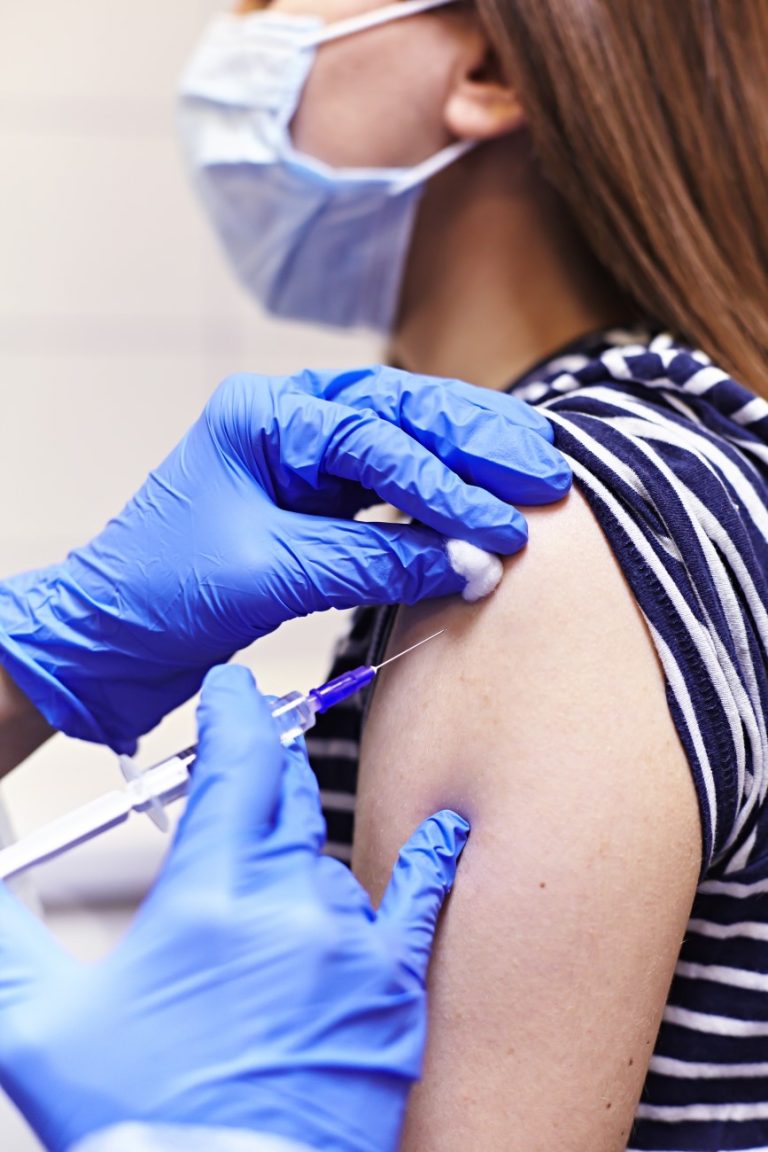
On Jul. 21, 2020, researchers from the National Institutes of Health (NIH) announced that early results from the…

On Jul. 21, 2020, researchers at Washington University School of Medicine in St. Louis and the Saint Louis…

On Jul. 21, 2020, BD (Becton, Dickinson) announced additional pandemic orders for needles and syringes from the U.S….
On Jul. 20, 2020, Valneva confirmed its participation in the UK government COVID-19 vaccine response program. Valneva had…

On Jul. 20, 2020, the Serum Institute of India (SII) announced that with the trials of the COVID-19…

On Jul. 20, 2020, Pfizer and BioNTech announced an agreement with the United Kingdom to supply 30 million…

On Jul. 20, 2020, BioNTech and Pfizer announced initial data from their ongoing German Phase 1/2, open-label, non-randomized,…
On Jul. 20, 2020, Thermo Fisher Scientific expanded global capacity and capabilities across its leading pharma services network…

On Jul. 20, 2020, scientists at HDT Bio, PAI Life Sciences, and the University of Washington announced they…

On Jul. 19, 2020, HDT Bio announced co-development efforts with Gennova Biopharmaceuticals to start evaluating HDT-301, a COVID-19…

On Jul. 16, 2020, Novartis announced a new initiative to help patients in low-income and lower-middle-income countries (LIC;…

On Jul. 16, 2020, Dynavax Technologies and the Icahn School of Medicine at Mount Sinai announced they had…

On Jul. 16, 2020, Tonix Pharmaceuticals announced it had entered into a research collaboration and option agreement with…
On Jul. 14, 2020, the WHO and UNICEF warned of an alarming decline in the number of children…

On Jul. 15, 2020, seventy-five countries submitted expressions of interest to protect their populations and those of other…

On Jul. 15, 2020, Tevogen Bio announced its intention to evaluate its proprietary antigen-specific T cell technology as…
On Jul. 14, 2020, an investigational vaccine, mRNA-1273, designed to protect against SARS-CoV-2, the virus that causes coronavirus…

On Jul. 14, 2020, researchers at the The University of Hong Kong (HKUMed) collaborated with researchers at the…

On Jul. 14, 2020, Shanghai Fosun Pharmaceutical announced it had received the acceptance notice of clinical trial application…

On Jul. 14, 2020, Moderna announced the publication of an interim analysis of the open-label Phase 1 study…

On Jul. 13, 2020, Altimmune announced positive results from the preclinical studies of its intranasal COVID-19 vaccine candidate,…

On Jul. 13, 2020, Tonix Pharmaceuticals announced a new preclinical research and option agreement with Kansas State University…
On Jul. 13, 2020, researchers from Virginia Tech and the NIH suggested that Bacille Calmette-Gu�rin (BCG), a tuberculosis…

On Jul. 9, 2020, Atos announced that the Biocomputing Unit at the Spanish National Center for Biotechnology (CNB),…

On Jul. 9, 2020 Moderna and Laboratorios Farmaceuticos Rovi announced a collaboration for large-scale, commercial fill-finish manufacturing of…

On Jul. 9, 2020, Altimmune announced that it had entered into a teaming agreement with DynPort Vaccine Company…

On Jul. 8, 2020, scientists at the UC Berkeley-UCSF Joint Medical Program reported that for some individuals with…

On Jul. 8, 2020, Kaiser Permanente Washington Health Research Institute (KPWHRI) announced that it had created a registry…

On Jul. 8, 2020, Moderna announced that it had completed enrollment for both cohorts of the Phase 2…
On Jul. 8, 2020, BD (Becton, Dickinson) announced the formation of a strategic, public-private partnership with the Biomedical…