Moderna announced supply agreement with the Philippines for 7 million additional doses of COVID-19 Vaccine Moderna
On Mar. 22, 2021, Moderna announced that the Philippines had secured 7 million additional doses of COVID-19 Vaccine…

On Mar. 22, 2021, Moderna announced that the Philippines had secured 7 million additional doses of COVID-19 Vaccine…

On Mar. 18, 2021, Incyte announced results from the Phase 3 DEVENT study evaluating the efficacy and safety…

On Mar. 17, 2021, the U.S. Department of Health and Human Services (HHS) announced the investment of $10…
On Mar. 16, 2021, research from the WHO and partners showed that the COVID-19 pandemic was severely affecting…

On Mar. 15, 2021, Twist Bioscience announced that it had started shipping its new synthetic RNA reference controls,…

On Mar. 11, 2021, Altimmune announced that it had expanded its previously-announced AdCOVID manufacturing collaboration with Lonza. Under…

On Mar. 11, 2021, ContraFect announced that it had been awarded a cost-share contract from the Biomedical Advanced…

On Mar. 10, 2021, Innovation Pharmaceuticals announced that a Machine Learning (Artificial Intelligence) model used to screen 1,482…

On Mar. 8, 2021, the National Institutes of Health (NIH) announced that it had launched the last of…

On Mar. 8, 2021, AXIM Biotechnologies announced that it had successfully completed point-of-care clinical trials on its much…

On Mar. 8, 2021, AIM ImmunoTech announced that it had dosed the first healthy subjects in its Phase…

On Mar. 5, 2021,Takeda Pharmaceutical announced that it has submitted a New Drug Application to the Government of…

On Mar. 5, 2021, Innovation Pharmaceuticals reported that eight sites were participating in the Company’s international Phase 2…

On Mar. 5, 2021, Merck and Ridgeback Biotherapeutics, announced preliminary results from Ridgeback’s Phase 2a randomized, double-blind, placebo-controlled…

On Mar. 4, 2021, researchers at the University School of Medicine in St. Louis announced that three new,…

On Mar. 3, 2021, Pfizer and BioNTech, in partnership with UNICEF, announced the arrival of the first doses…

On Mar. 2, 2021, the National Institutes of Health (NIH) announced it had launched the next version of…

On Mar. 2, 2021, the National Institutes of Health (NIH), announced that it had launched a research effort…

On Mar. 2, 2021, Innovation Pharmaceuticals reported that eight sites were participating in the Companyメs international Phase 2…

On Feb. 25, 2021, the U.S. Food and Drug Administration (FDA) granted approval to Sarepta Therapeutics for Amondys…

On Feb. 24, 2021, NeuroRx, announced that the phase 2b/3 trial of ZYESAMI for the treatment of Respiratory…

On Feb. 19, 2021, BioNTech announced the submission of new data to the FDA demonstrating the stability of…

On Feb. 19, 2021, Luminex announced that it had received $11.3 million in funding from the Biomedical Advanced…

On Feb. 18, 2021, scientists at the University of Oxford announced that the Randomised Evaluation of COVID-19 Therapy…
On Feb. 17, 2021, Novartis announced that it had entered into a grant agreement with the Bill &…

On Feb. 17, 2021, Altimmune announced that the U.S. Food and Drug Administration (FDA) had cleared the Companyls…

On Feb. 17, 2021, Immunic announced that its lead asset, IMU-838, the company’s selective oral DHODH inhibitor, had…

On Feb. 17, 2021, BioNTech announced results from an in vitro study that provided additional data on the…

On Feb. 16, 2021, Lonza, formerly Bend Research, announced that it was expanding and refining first-in-human services at…
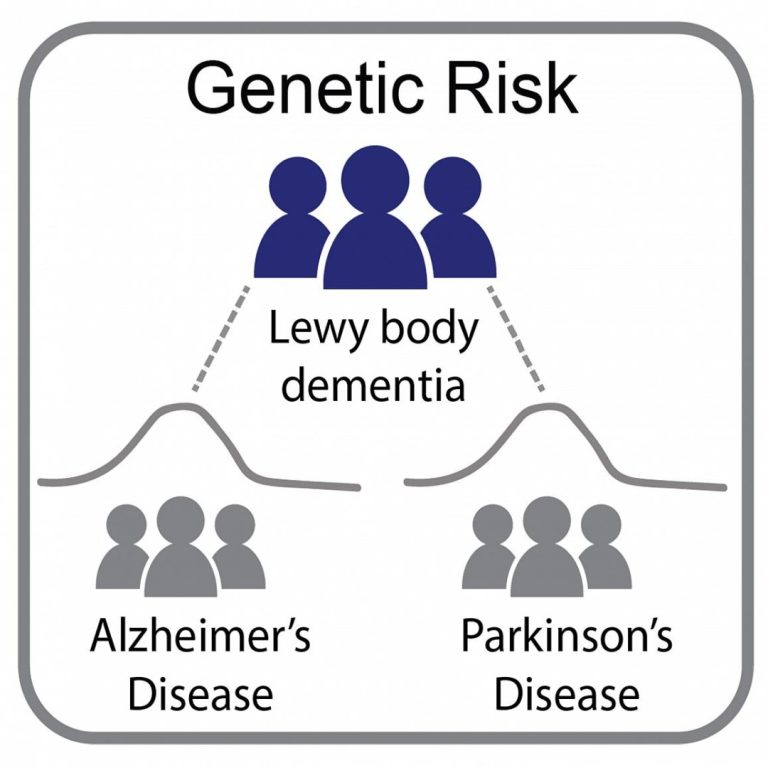
On Feb. 16, 2021, in a study led by National Institutes of Health (NIH) researchers, scientists found that…