Moderna announced agreement with Taiwan for 20 million doses of Moderna’s COVID-19 Vaccine and variant booster
On Jul. 22, 2021, Moderna announced a new supply agreement with Taiwan for 20 million doses of Moderna’s…
On Jul. 22, 2021, Moderna announced a new supply agreement with Taiwan for 20 million doses of Moderna’s…
On Jul. 16, 2021, Merck announced the U.S. Food and Drug Administration (FDA) had approved VAXNEUVANCE (Pneumococcal 15-valent…
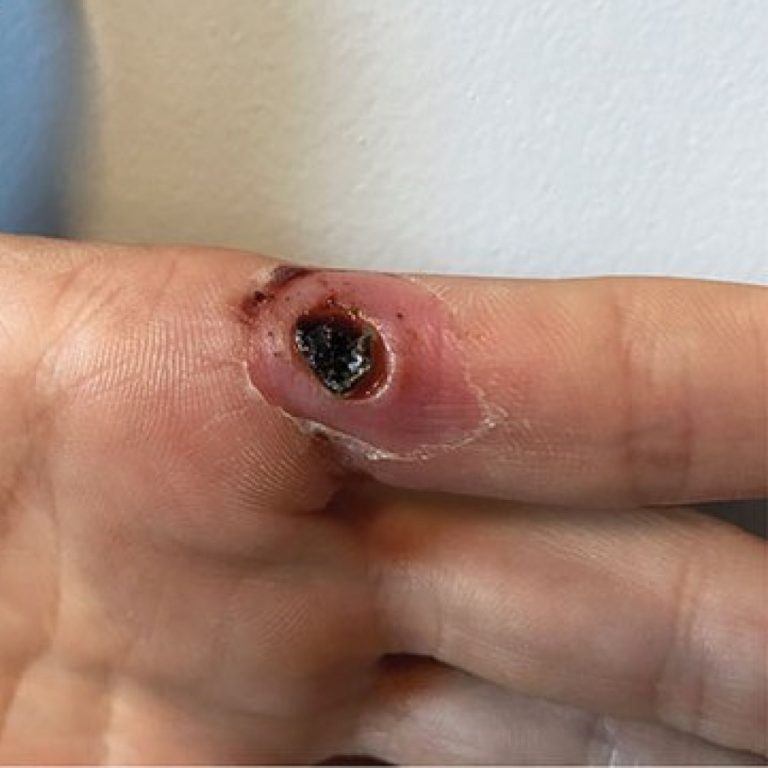
On Jul. 16, 2021, the U.S. Centers for Disease Control and Prevention (CDC) announced and the Texas Department…
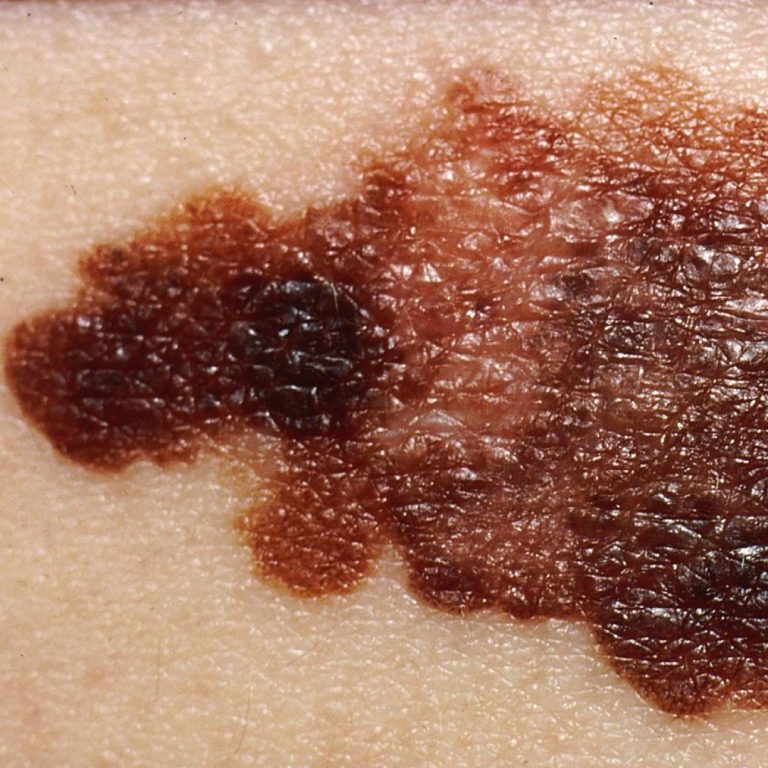
On Jul. 8, 2021, the National Institutes of Health (NIH) reported that the overall cancer death rates continue…

On Jul. 8, 2021, access to the world’s largest browsable resource linking rare protein-coding genetic variants to human…
On Jun. 30, 2021, SIGA Technologies announced that it had provided TPOXX (tecovirimat) to Liverpool University Hospitals NHS…
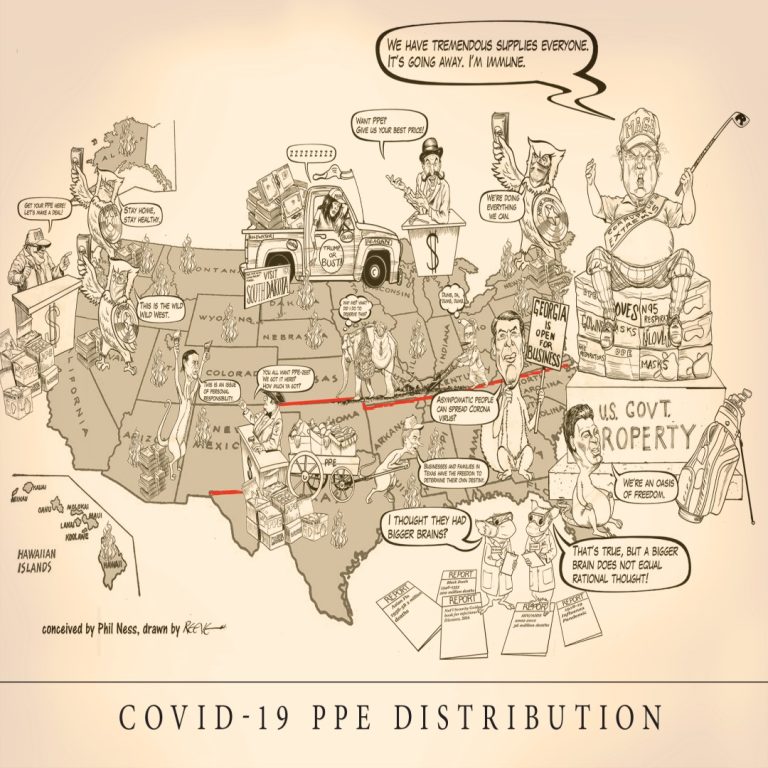
Cast of Characters: Jay Inslee, Governor, Washington | Gavin Newsom, Governor, California | Doug Ducey, Governor, Arizona |…

On Jun. 23, 2021, Biogen and Eisai announced that the U.S. Food and Drug Administration (FDA) had granted…

On Jun. 22, 2021, the National Research Council of Canada (NRC) announced it had completed construction of the…

On Jun. 21, 2021, Gilead Sciences announced positive data from three retrospective studies of the real-world treatment of…

On Jun. 21, 2021, Tonix Pharmaceuticals announced that it planned to develop TNX-102 SL (cyclobenzaprine HCl sublingual tablets)…
On Jun. 17, 2021, the U.S. Food and Drug Administration (FDA) Center for Biologics Evaluation and Research granted…

On Jun. 16, 2021, Regeneron announced positive results from the largest trial assessing any monoclonal antibody treatment in…

On Jun. 15, 2021, Moderna and Magenta Investments, an industrial conglomerate in the United Arab Emirates (UAE), announced…

On Jun. 14, 2021, University of British Columbia researchers Dr. Jayachandran Kizhakkedathu, Dr. Caigan Du and Dr. Christopher…
On Jun. 14, 2021, Cocrystal Pharma announced the selection of CDI-45205 as the lead compound for further development…

On Jun. 11, 2021, Moderna and Tabuk Pharmaceutical announced an agreement to commercialize the Moderna COVID-19 Vaccine and…

On Jun. 8, 2021, Twist Bioscience announced that it had started shipping its synthetic RNA reference controls for…

On Jun. 8, 2021, Mayo Clinic researchers and colleagues at the University of Minnesota showed that COVID-19 exacerbates…

On Jun. 7, 2021, MediciNova announced it had initiated a sheep study to investigate MN-166 (ibudilast) in an…

On Jun. 7, 2021, Biogen and Eisai announced that the U.S. Food and Drug Administration (FDA) had granted…

On Jun. 4, 2021, Regeneron announced the U.S. Food and Drug Administration (FDA) updated the Emergency Use Authorization…

On Jun. 1, 2021, CytoDyn announced that Chiral Pharma in the Philippines placed its first purchase order for…

On Jun. 1, 2021, the U.S. Department of Health and Human Services (HHS) unveiled a new type of…

On May 28, 2021, Amgen announced that the U.S. Food and Drug Administration (FDA) had approved LUMAKRAS (sotorasib)…

On May 28, 2021, Pfizer and BioNTech announced that the Conditional Marketing Authorization for COMIRNATY in the European…

On May 27, 2021, Innovation Pharma announced that patient enrollment in the Company’s 120-patient, Phase 2 clinical trial…
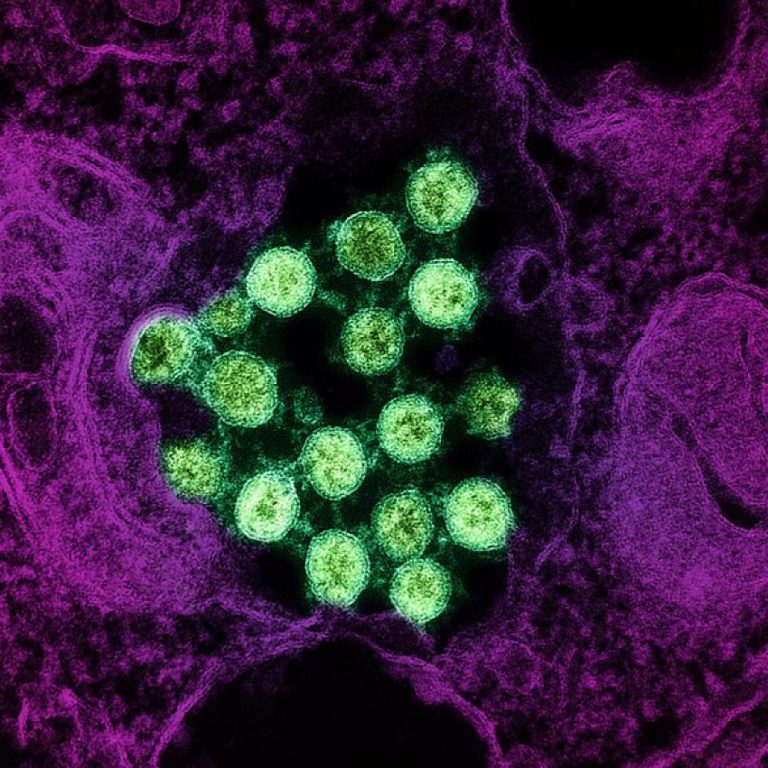
On May 27, 2021, Seattle Children’s researchers published a study that uncovered a deeper understanding of why people…
On May 26, 2021, National Institutes of Health (NIH) scientists reported that the immune system’s attempt to eliminate…
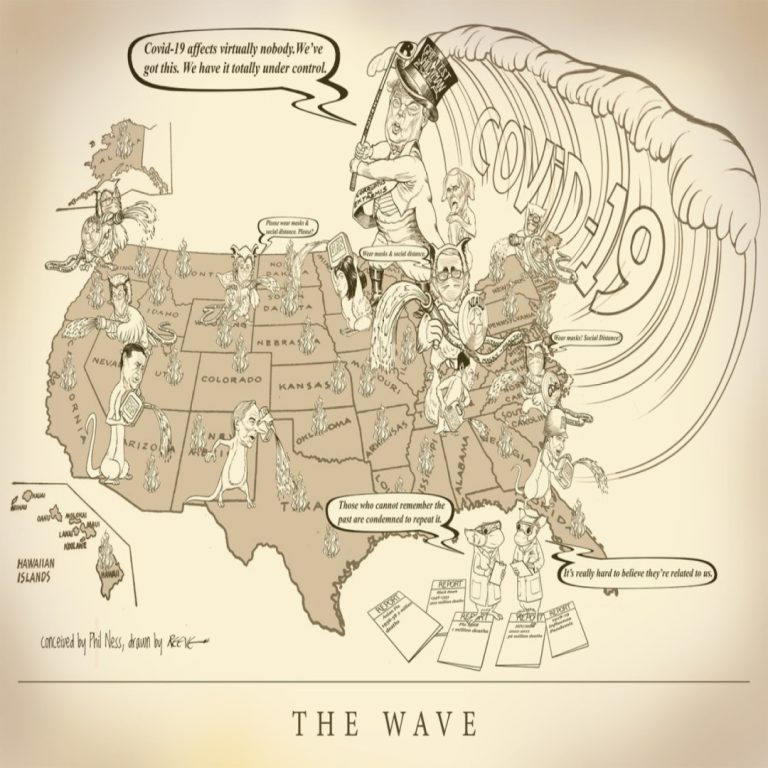
Cast of Characters: Donald Trump, President | Mike Pence, Vice President | Anthony S. Fauci, MD, Director, National…