Sorrento announced FDA authorization to proceed with phase 1 study of Intranasal STI-9199 against Covid-19 viruses
On Mar. 2, 2022, Sorrento Therapeutics announced that it had received clearance from the FDA for its investigational…

On Mar. 2, 2022, Sorrento Therapeutics announced that it had received clearance from the FDA for its investigational…

On Feb. 28, 2022, the Patent and Trial Appeal Board of the U.S. Patent and Trademark Office (USPTO)…

On Feb. 22, 2022, Sorrento Therapeutics announced that additional preclinical results demonstrate broad spectrum COVISHIELD (STI-9167) neutralizing activity…

On Feb. 1, 2022, University of Virginia Cancer Center announced it had been awarded a Comprehensive Cancer Center…

On Feb. 17, 2022, after studying blood samples from 244 patients hospitalized for COVID-19, a group of researchers,…

On Feb. 17, 2022, Resverlogix announced that the first Brazilian site had been initiated for its Phase 2b…
On Feb. 15, 2022, a woman with HIV who received a cord blood stem cell transplant to treat…
On Feb. 12, 2022, China’s medical products regulator announced that it had given conditional approval for Pfizer’s COVID-19…
On Feb. 11, 2022, Gilead Sciences announced new data from an interim analysis of its ongoing, Phase 2/3…

On Feb. 11, 2022, Gilead Sciences announced new data from an interim analysis of its ongoing, Phase 2/3…

On Feb. 8, 2022, Merck and Ridgeback Biotherapeutics announced that a total of 3.1 million courses of molnupiravir,…
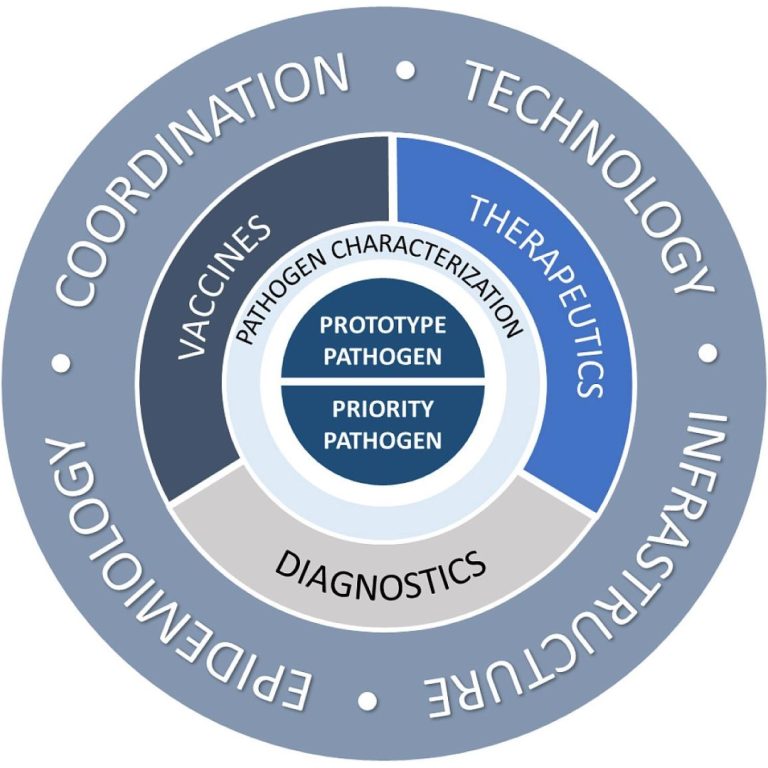
On Feb. 2, 2022, NIAID scientists announced the new Pandemic Preparedness Plan aimed to support critical basic and…

On Feb. 2, 2022, a new analysis of the first two patients treated in a clinical trial with…
On Feb. 2, 2022, President Biden announced a reignition of the Cancer Moonshot, that highlighted new goals: to…

On Jan. 28, 2022, Merck and Ridgeback Biotherapeutics announced data from six preclinical studies demonstrating that molnupiravir, an…

On Jan. 27, 2022, Cocrystal Pharma announced that it had selected two investigational novel antiviral drug candidates for further…

On Jan. 26, 2022, a former University of British Columbia post-doctoral research fellow led an international research team…

The Evolution of CRISPR illustrates its climb out of the primordial ocean on the backs of eukaryotic cells…

On Jan. 24, 2022, Atara Biotherapeutics, a leader in T-cell immunotherapy, announced that it had leveraged its novel…

On Jan. 20, 2022, scientists from the National Institute of Allergy and Infectious Diseases (NIAID) announced that they…

On Jan. 20, 2022, a clinical trial funded by the National Institutes of Health (NIH) found that giving…

On Jan. 18, 2022, Merck and Ridgeback Biotherapeutics announced the signing of a long-term supply agreement with the…

On Jan. 12, 2022, Pfizer announced positive top-line results from a Phase 3 study describing the safety and…

On Jan. 4, 2022, Pfizer announced that the U.S. government had committed to purchasing an additional 10 million…

Our Heroes and Remembrance illustration has Maurice Ralph Hilleman and John Enders, pioneering developers of common vaccines, and…

On Dec. 24, 2021, Merck and Ridgeback Biotherapeutics announced that Japanメs Ministry of Health, Labor and Welfare had…

On Dec. 23, 2021, Merck and Ridgeback Biotherapeutics announced that the U.S. Food and Drug Administration (FDA) had…

On Dec. 23, 2021, Pfizer and BioNTech announced that they had submitted longer-term follow-up data from the companiesメ…

On Dec. 22, 2021, Gilead Sciences announced full results from a Phase 3 investigational study evaluating the efficacy…
On Dec. 22, 2021, Cocrystal Pharma announced that in vitro studies demonstrate its oral and intranasal/pulmonary SARS-CoV-2 main…