Milestones in Polio
Milestones in Polio illustrates the slow but steady advancements from its clinical description and discovery to widespread infection…

Milestones in Polio illustrates the slow but steady advancements from its clinical description and discovery to widespread infection…

On Dec. 24, 2022, the U.S. Food and Drug Administration (FDA) approved updated labeling for Genentech’s capecitabine tablets…
On Dec. 22, 2022, Gilead Sciences announced that Sunlenca (lenacapavir), in combination with other antiretroviral(s) (ARV), had been…

On Dec. 13, 2022, Texas Biomed announced that it had been designated as a prime contractor by the…

On Dec. 10, 2022, CSL announced data affirming the long-term durability and safety of single-infusion HEMGENIX® (etranacogene dezaparvovec-drlb)…

On Dec. 9, 2022, the World Health Organization (WHO) announced that the first doses of one of the…
On Dec. 9, 2022, the U.S. Food and Drug Administration (FDA) approved Genentech’s atezolizumab (Tecentriq) for adult and…

On Dec. 7, 2022, Novavax announced that Health Canada had approved a supplement to a New Drug Submission…
On Dec. 5, 2022, the Fred Hutchinson Cancer Center announced that according to a new meta-analysis women were…

On Nov. 22, 2022, the U.S. Food and Drug Administration approved the investigational gene therapy etranacogene dezaparvovec or…

On Nov. 17, 2022, the U.S. Food and Drug Administration (FDA) approved Tzield (teplizumab-mzwv) injection to delay the…

On Nov. 16, 2022, the Cancer Prevention and Research Institute of Texas (CPRIT) approved $12 million in recruitment…

On Nov. 16, 2022, researchers at the National Institutes of Health (NIH) announced they had successfully identified differences…

On Nov. 15, 2022, Moderna reported findings from a Phase II/III clinical trial where bivalent Omicron-targeting booster candidates,…
On Nov. 14, 2022, in a study published in Nature Communications, researchers discovered the pathway by which chronic…

On Nov. 9, 2022, Novavax announced that the Medicines and Healthcare products Regulatory Agency (MHRA) in the United…
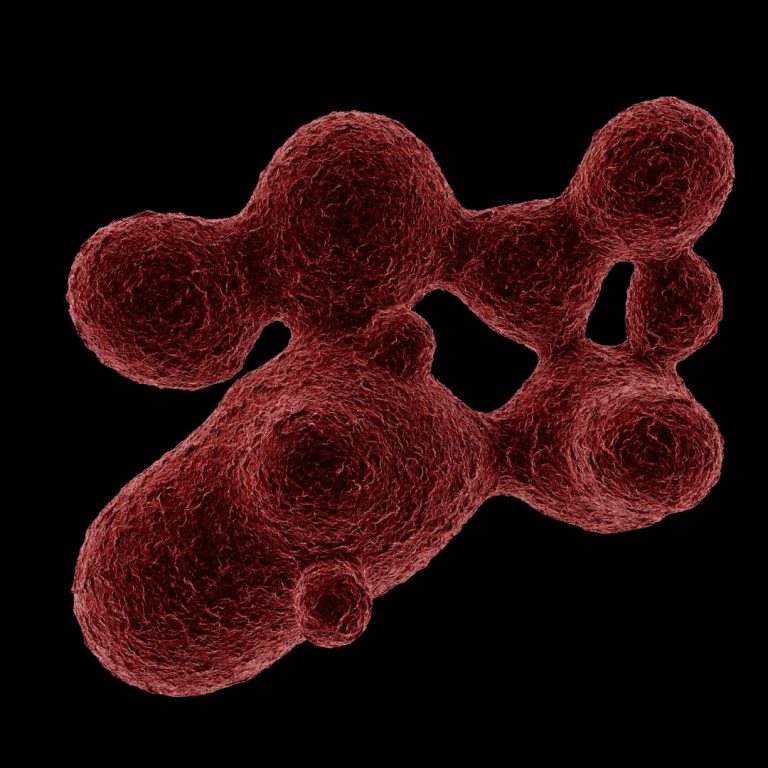
On Nov. 7, 2022, the NHS Blood and Transplant (NHSBT) reported that red blood cells grown in a…
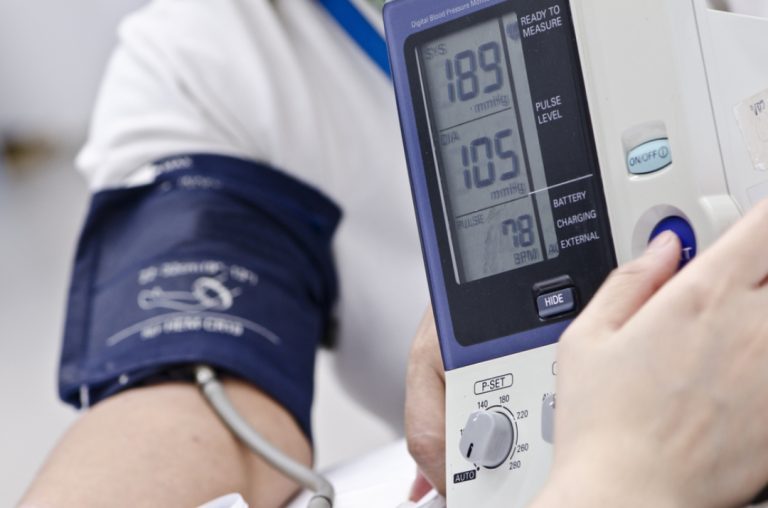
On Nov. 1, 2022, the National Institutes of Health (NIH) announced that adults with hypertension saw a small,…

On Oct. 27, 2022, the National Institutes of Health (NIH) announced that overall cancer death rates continued to…

On Oct. 26, 2022, the Barbara Ann Karmanos Cancer Institute announced it had completed an over 50,000-square-foot expansion…
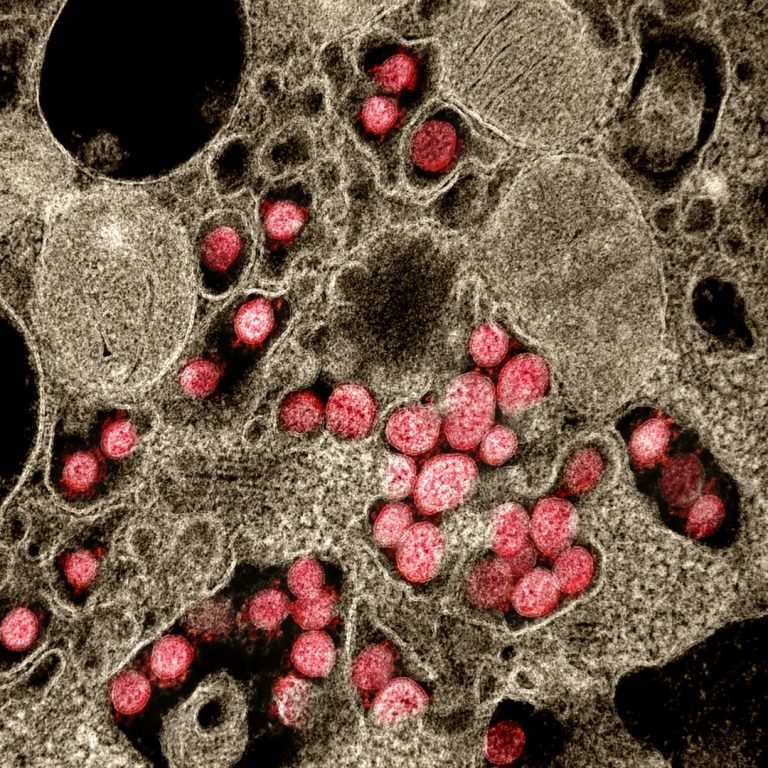
On Oct. 25, 2022, Pacific Northwest National Laboratory (PNNL) announced that scientists have shown that they can detect…

On Oct. 19, 2022, the U.S. Food and Drug Administration final rule establishing Over-the-Counter Hearing Aids to improve…

On Oct. 13, 2022, Pfizer and BioNTech announced early data from a Phase 2/3 clinical trial (NCT05472038) evaluating…

On Oct. 12, 2022, the Fred Hutchinson Cancer Center (FHCRC) announced the Bezos family had committed $710.5 million…
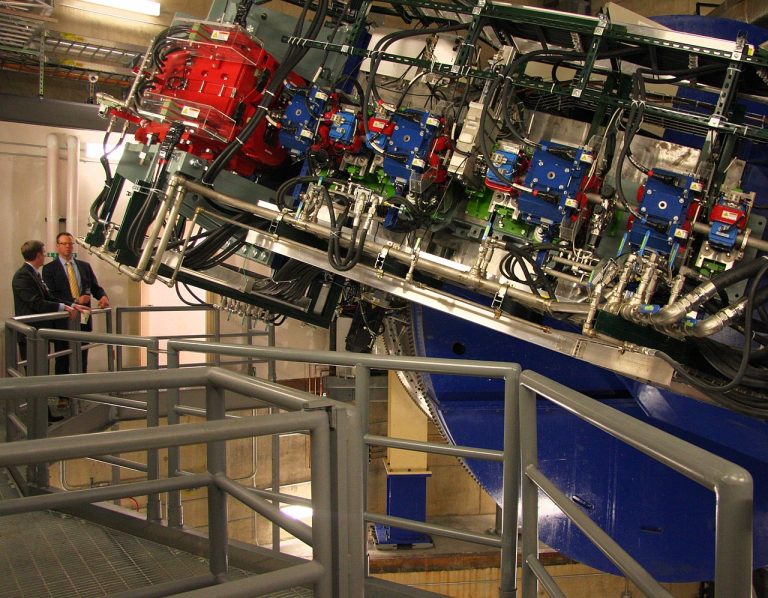
On Oct. 10, 2022, the Mayo Clinic announced that The Fred C. and Katherine B. Andersen Foundation of…

On Oct. 7, 2022, Pfizer and BioNTech announced that Health Canada had authorized COMIRNATY Original & Omicron BA.4/BA.5…
On Sept. 27, 2022, the National Institutes of Health (NIH) announced it had launched a program to better…

On Supt. 22, 2022, Pfizer announced an agreement to supply up to six million treatment courses of its…
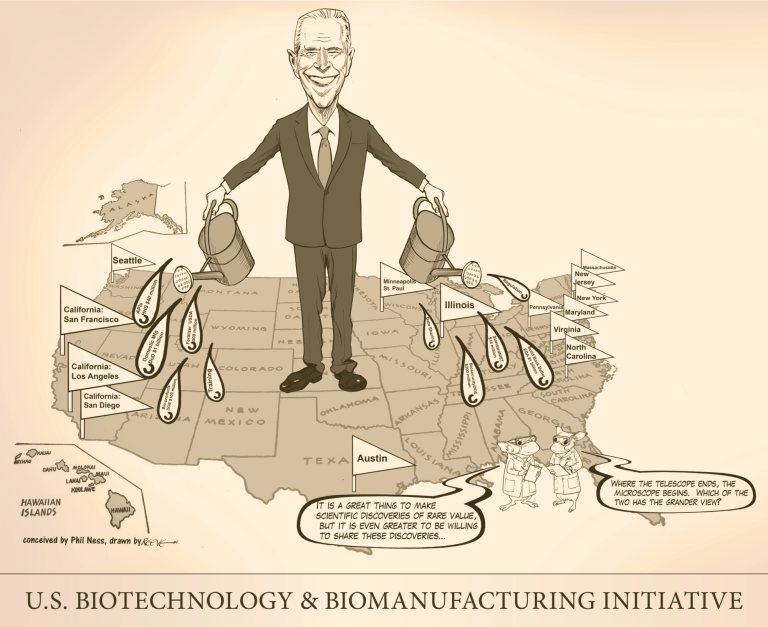
On Sept. 14, 2022, The U.S. Biotech Initiative was announced detailing new investments and resources to advance the…

On Sept. 8, 2022, the Fred Hutchinson Cancer Center (FHCRC) announced that Stuart and Molly Sloan had pledged…