Small robot made big difference for cochlear implant patient at Oregon Health & Science University
On Jan. 24, 2024, Oregon Health & Science University announced that Pamela Runner, 77, of Corvallis, became the…
On Jan. 24, 2024, Oregon Health & Science University announced that Pamela Runner, 77, of Corvallis, became the…

On Jan. 16, 2024, the U.S. Food and Drug Administration (FDA) announced it had approved CRISPR Therapeutics’ CASGEVY…

On Jan. 15, 2024, CSL announced that Swissmedic had authorised HEMGENIX® (etranacogene dezaparvovec), the first and currently only gene…

On Jan. 10, 2024, an National Institutes of Health (NIH) supported study announced an n atlas revealing the…

On Jan. 9, 2024, Biogen and Eisai announced that humanized anti- soluble aggregated amyloid-beta (Aβ) monoclonal antibody ‘LEQEMBI’…
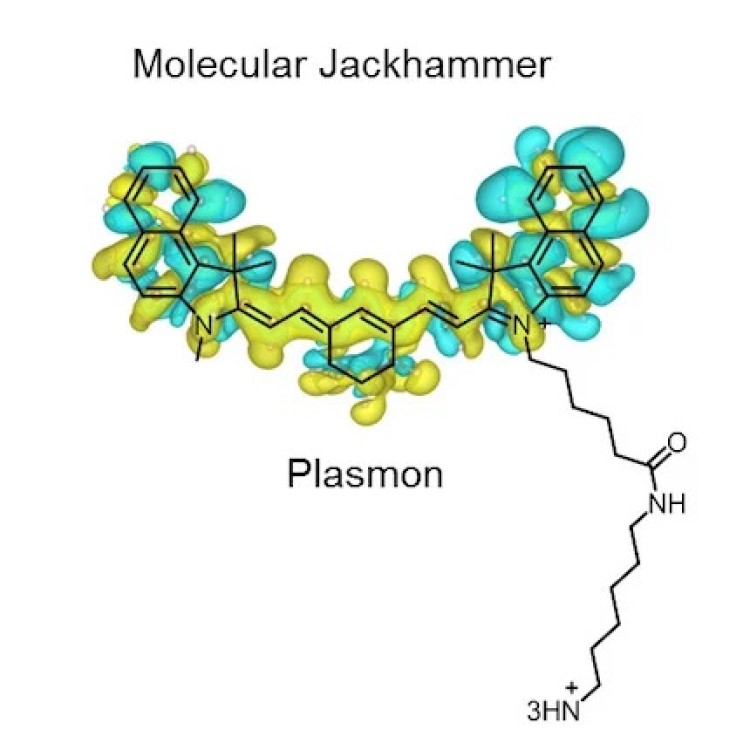
On Dec. 19, 2023, a research team led by Rice University reported they found that the atoms of…

On Dec. 14, 2023, the Department of Energy’s (DOE) Pacific Northwest National Laboratory (PNNL) announced that it had…

On Dec. 14, 2023, the University of Alberta announced that a study published in Nature sheds light on…

On Dec. 14, 2023, Biogen and Sage Therapeutics announced ZURZUVAE (zuranolone) 50 mg (two 25 mg capsules per…
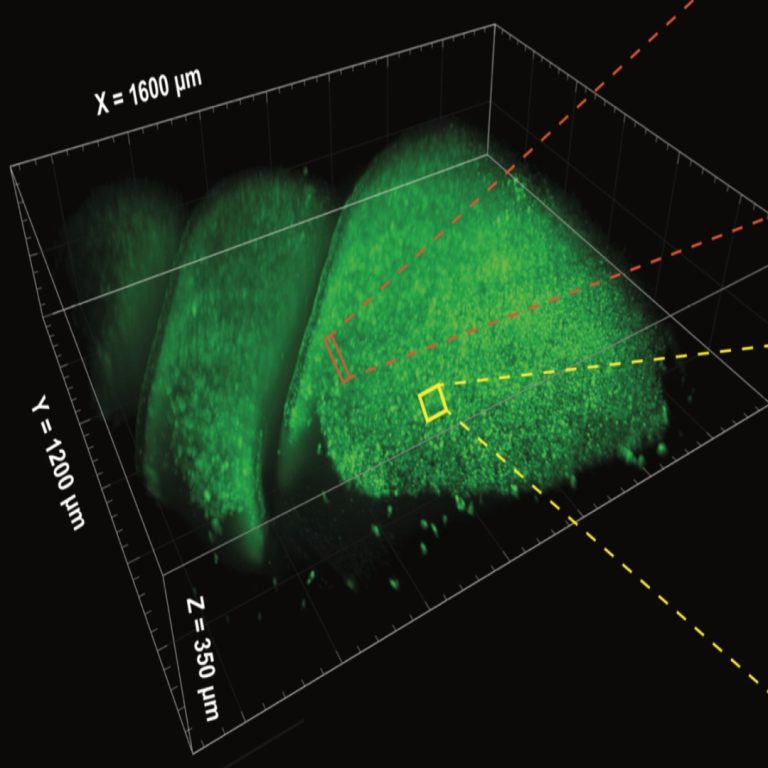
On Dec. 13, 2023, for the first time ever, an international team of researchers announced they had created…
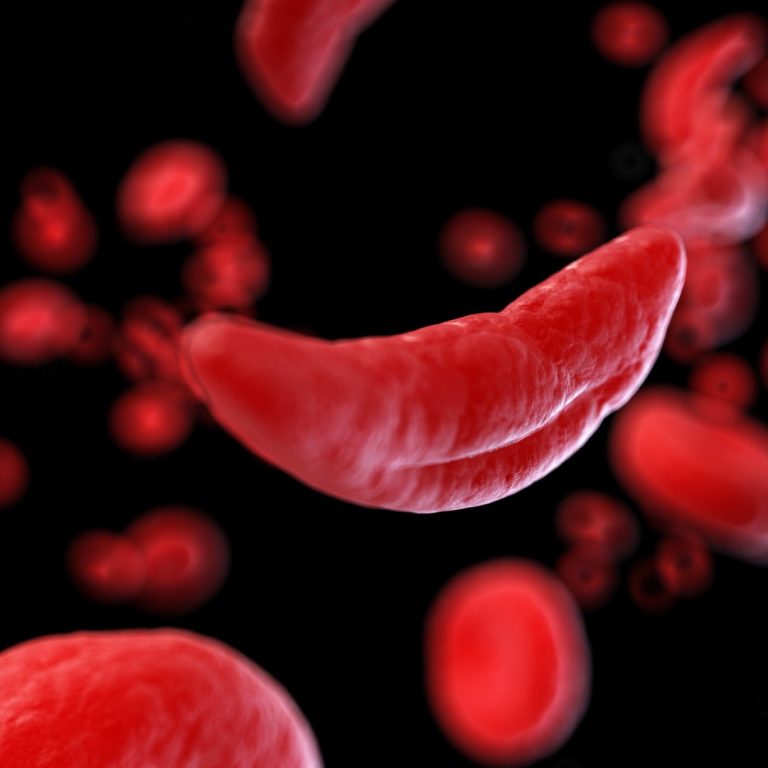
On Dec. 8, 2023, the U.S. Food and Drug Administration (FDA) approved two milestone treatments, Casgevy and Lyfgenia,…

On Dec. 8, 2023, Vertex Pharmaceuticals and CRISPR Therapeutics announced that the U.S. Food and Drug Administration (FDA)…

On Dec. 8, 2023, bluebird bio announced the U.S. commercial launch of its LYFGENIA’ (lovotibeglogene autotemcel, also known…

On Dec. 4 2023, the University of Wisconsin-Madison researchers announced that while analyzing data from wastewater samples collected…

On Nov. 30, 2023, in a momentous landmark for medical research, UK Biobank announced that it had unveiled…

On Nov. 22, 2023, the U.S. Food and Drug Administration (FDA) issued the final guidance COVID-19: Developing Drugs…

On Nov. 16, 2023, Vertex and CRISPR Therapeutics announced that the United Kingdom (U.K.) Medicines and Healthcare products…

On Nov. 15, 2023, the Cancer Prevention and Research Institute of Texas (CPRIT) announced that it had approved…

On Nov. 13, 2023, the World Health Organization (WHO announced that the Ministry of Health and Social Welfare…

On Nov. 13, 2023, the White House announced the first-ever Initiative on Womenメs Health Research, an effort led…
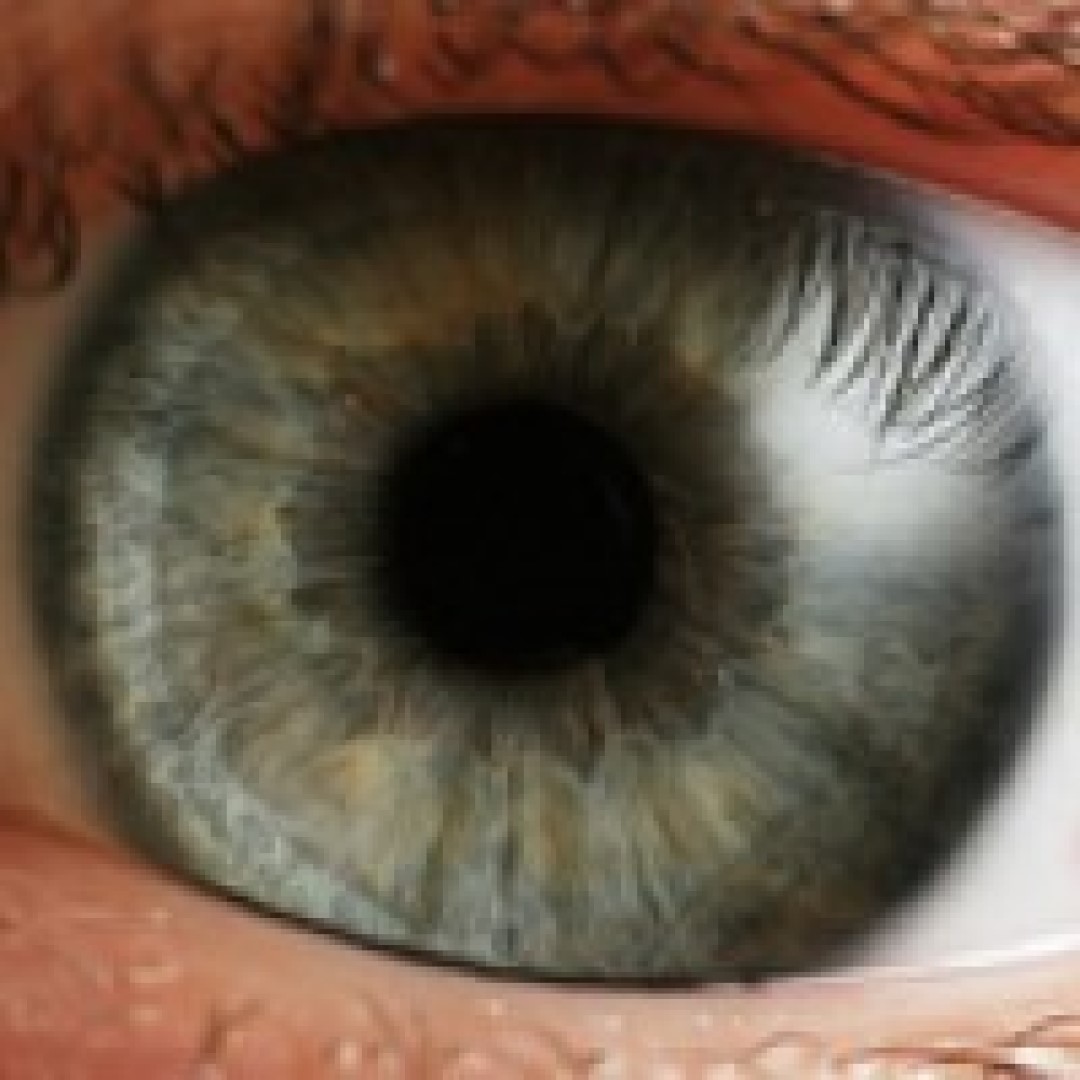
On Nov. 10, 2023, surgeons in New York announced they had performed the first-ever whole-eye transplant in a…

On Nov. 8, 2023, the U.S. Food and Drug Administration approved Eli Lilly’s Zepbound (tirzepatide) injection for chronic…

On Oct. 31, 2023, the U.S. Food and Drug Administration (FDA) announced it had approved Amgen’s Wezlana (ustekinumab-auub)…
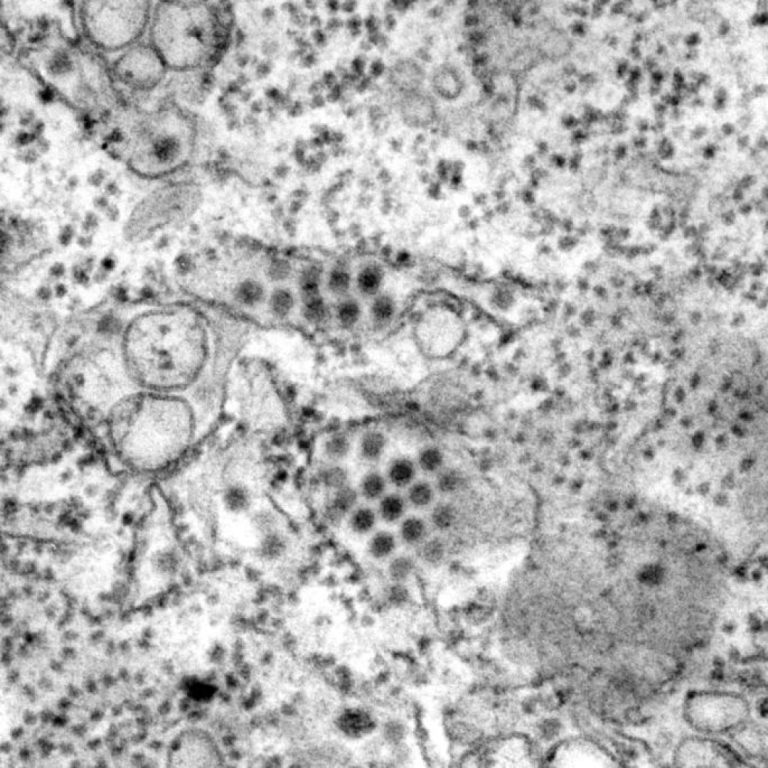
On Oct. 30, 2023, the California Department of Public Health (CDPH) reported the first locally acquired case of…
On Oct. 27, 2023, the California Institute for Regenerative Medicine (CIRM) awarded $42.1 million to fund various translational…

On Oct. 20, 2023, the U.S. Centers for Disease Control and Prevention (CDC) reported that among children and…

On Oct. 14, 2023, Purdue University ensured that the memory of the former graduate and devoted Boilermaker football…

On Oct. 2, 2023, the California Institute for Regenerative Medicine (CIRM) announced its approval of a new program…

On Oct. 2, 2023, the Nobel Prize in Physiology or medicine was awarded jointly to Katalin Kariko and…

On Sept. 29, 2023, through the Future Manufacturing program, the U.S. National Science Foundation (NSF) announced targeted investments…