E. coli Outbreak Linked to McDonald’s Quarter Pounders
On Oct. 22, 2024, the U.S. Centers for Disease Control (CDC) announced that public health officials in multiple…
On Oct. 22, 2024, the U.S. Centers for Disease Control (CDC) announced that public health officials in multiple…
On Oct. 21, 2024, in one of the largest-ever studies of DNA and brain volume, researchers announced they…
On Oct. 21, 2024, Rice University’s Baker Institute for Public Policy reported that hospital service prices surged more…

On Oct. 20, 2024, the Washington State Department of Health announced that four agricultural workers tested positive for…

On Oct. 18, 2024, a study published in JAMA Ophthalmology, found that approximately 4.22 million people in the…

On Oct. 18, 2024, a research team led by Radboud university medical center reported that a new PET…
On Oct. 17, 2024, Geisinger announced results of a study that showed increased risk for autism appears to…
On Oct. 16, 2024, researchers from the Wellcome Sanger Institute, Newcastle University and their collaborators announced they had…

On Oct. 16, 2024, Sanofi announced it will contribute $18 million to three Historically Black Medical Schools to…

On Oct. 14, 2024, researchers have found a link between two human-specific genes and the gene SYNGAP1, a…

On Oct. 14, 2024, Lundbeck and Longboard Pharmaceuticals announced an agreement for Lundbeck to acquire Longboard for payment…

On Oct. 14, 2024, the Global Preparedness Monitoring Board (GPMB) released a report that outlined 15 key drivers…
On Oct, 14, 2024, a team of researchers from the Allen Institute of Brain Science and the University…

On Oct. 14, 2024, RedHill Biopharma announced that the U.S. government’s Biomedical Advanced Research and Development Authority (BARDA)…
On Oct. 11, 2024, the World Health Organization (WHO) reported that a total of 58 cases of Marburg…
On Oct. 11, 2024, scientists at the Korea Advanced Institute of Science and Technology (KAIST) announced they had…
On Oct. 10, 2024, the U.S. Centers for Disease Control and Prevention announced that a 2023 presumed outbreak…

On Oct. 10, 2024, Genentech announced today that the U.S. Food and Drug Administration (FDA) approved ItovebiTM (inavolisib),…

On Oct. 9, 2024, a study led by Johns Hopkins Medicine researchers concluded that commonly used ways of…
On Oct. 9, 2024, researchers from Brigham and Women’s Hospital found that people with wide-ranging long COVID symptoms…
On Oct. 9, 2024, scientists from Oregon Health & Science University (OHSU) released a study that used imaging…

On Oct. 9, 2024, the Nobel Foundation awarded the Nobel Prize in Chemistry: one half to David Baker,…

Oct. 9, 2024, the Seattle Fire Department (SFD) expanded its Buprenorphine Pilot Program and Seattle became the first…

On Oct. 8, 2024, a team of University of Essex researchers found that the secret to losing weight…
On Oct. 7, 2024, Columbia University researchers announced they had developed a new optical pooled screening approach called…

On Oct. 7, 2024, The Nobel Foundation announced that Victor Ambros from Harvard University and Gary Ruvkun from…
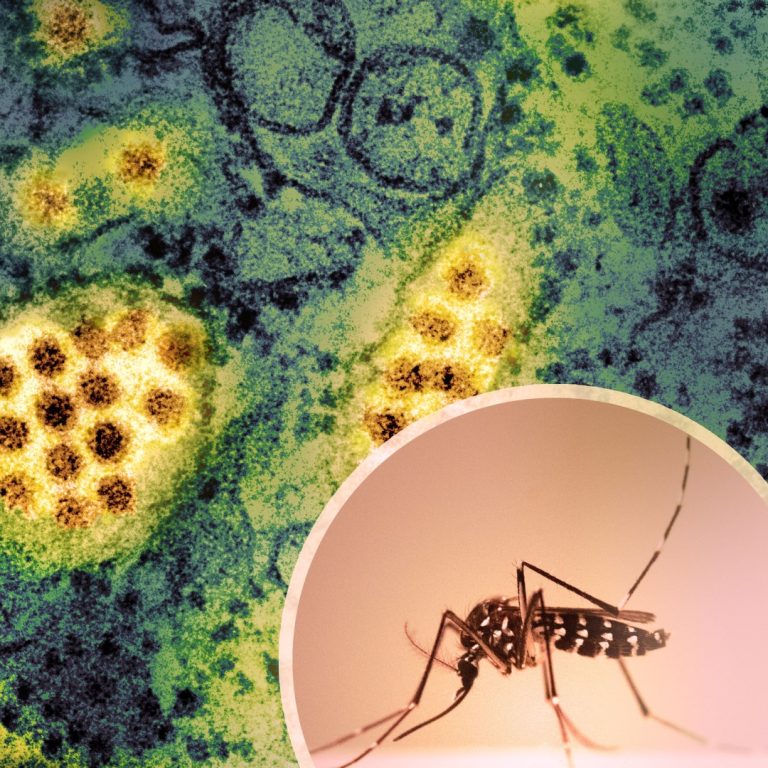
On Oct. 6, 2024, San Diego County officials announced they were investigating the first ever case of locally…

On Oct. 4, 2024, the Centers for Disease Control and Prevention (CDC) published data that showed older adults…

On Oct. 3, 2024, the National Health Service England (NHS) announced that Hundreds of babies have begun to…

On Oct. 3, 2024,the World Health Organization (WHO) launched the Global Strategic Preparedness, Readiness and Response Plan (SPRP)…