Marburg virus Outbreak Declared Ended in Tanzania
On Mar. 13, 2025, after two consecutive incubation periods (a total of 42 days) without a new confirmed…
On Mar. 13, 2025, after two consecutive incubation periods (a total of 42 days) without a new confirmed…

On Mar. 11, 2025, researchers from Texas A&M University School of Public Health report findings show significant links…

On Mar. 11, 2025, researchers at St Vincent’s Hospital Sydney announced the first implant of a BiVACOR Total…

On Mar. 10, 2025, Beam Therapeutics announced initial safety and efficacy data from its Phase 1/2 trial of…

On Mar. 9, 2025, Celltrion announced the U.S. Food and Drug Administration (FDA) approved OMLYCLO® (omalizumab-igec) as the first and…

On Mar. 7, 2025, a team of researchers from Aalto University and the University of Bayreuth announced that…
On Mar. 6, 2025, Cornell researchers announced they have discovered a new mechanism that genes use to survive…

On Mar. 6, 2025, researchers at the University of Cambridge announced they have discovered genes linked to obesity…
On Mar. 6, 2025, the U.S. Food and Drug Administration (FDA) announced the approval of ENCELTO (revakinagenetaroretcel-lwey), a…
On Mar. 4, 2025, researchers at a Mass Eye and Ear Institute-led trial announced taking stem cells from…

On Mar. 3, 2025, the Institute for Health Metrics and Evaluation reported that without urgent policy reform and…
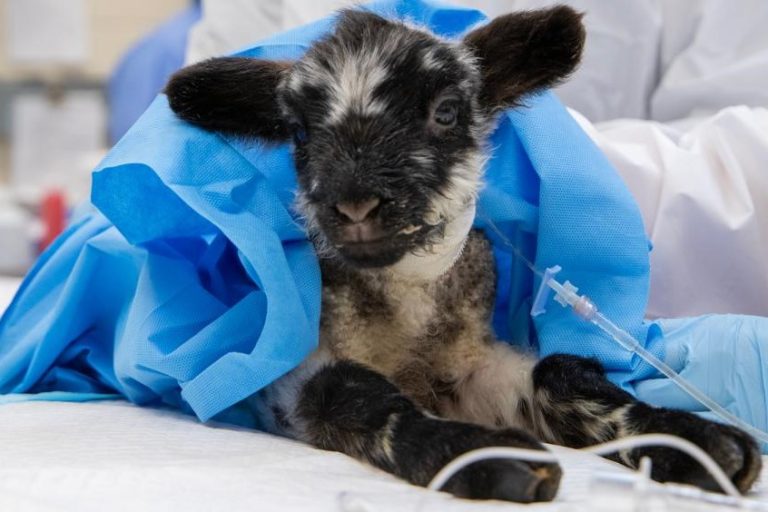
On Mar. 3, 2025, researchers from Oregon State University (OSU) announced the world’s first procedure successfully fixes lamb’s…

On Mar. 3, 2025, Genentech announced that the U.S. Food and Drug Administration (FDA) has approved TNKase® (tenecteplase),…

On Mar. 3, 2025, researchers at Fred Hutch Cancer Center announced they have discovered an overlooked mechanism driving…

On Feb. 27, 2025, ALK announced that the U.S. Food and Drug Administration (FDA) has approved ALK’s ODACTRA®…

In Feb. 26, 2025, Eli Lilly announced that it plans to bolster its domestic medicine production across therapeutic…

On Feb. 24, 2025, breast cancer is the most common cancer in women worldwide, but survival chances vary…

On Feb. 21, 2025, Novo Nordisk announced that the U.S. Food and Drug Administration (FDA) has determined the…
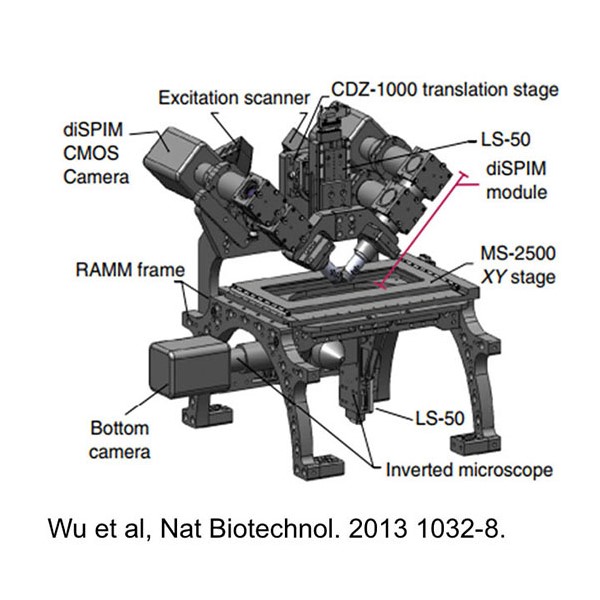
On Feb. 21, 2025, a team of researchers from the Marine Biological Laboratory (MBL) announced a hybrid custom-designed…

On Feb. 20, 2025, researchers from the University of Washington and Seattle Children’s Research Institute announced findings from…
On Feb. 19, 2025, researchers led by University of Virginia’s (UVA) Lu Q. Le, MD, PhD, announced they…

On Feb. 19, 2025, the Cancer Prevention and Research Institute of Texas (CPRIT) announced that it had approved…

On Feb. 19, 2025, Alcon announced the full U.S. commercial availability of Voyager™ DSLT, the first and only…

On Feb. 19, 2025, research from the Texas A&M College of Veterinary Medicine and Biomedical Sciences has discovered…
On Feb. 19, 2025, scientists at St. Jude Children’s Research Hospital report results from a promising new approach…

On Feb. 17, 2025, researchers from Texas Children’s Hospital in Houston announced that the four year old girl…

On Feb. 17, 2025, Stanford researchers have conducted the first large-scale screen of these inherited changes, called single…

On Feb. 14, 2025, the World Health Organization (WHO) announced that a cumulative of two confirmed and eight…
On Feb. 13, 2025, researchers at Washington University School of Medicine in St. Louis announced a new analysis…
On Feb. 12, 2025, researchers at the Max Planck Institute released a study that could pave the way…