COVID-19 Wheel of Fortune
Play the COVID-19 Wheel of Fortune and see how lucky you are! You have two wheels to choose…
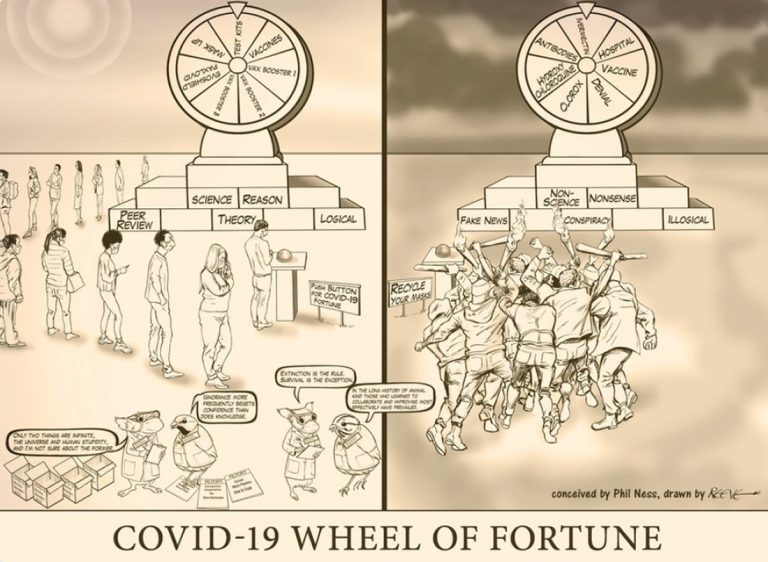
Play the COVID-19 Wheel of Fortune and see how lucky you are! You have two wheels to choose…

On Jun. 15, 2022, Pfizer and BioNTech announced the European Medicines Agency (EMA) had initiated a rolling review…

On Mar. 29, 2022, Pfizer and BioNTech announced that the U.S. Food and Drug Administration (FDA) had expanded…

On Feb. 22, 2022, Sorrento Therapeutics announced that additional preclinical results demonstrate broad spectrum COVISHIELD (STI-9167) neutralizing activity…

On Feb. 15, 2022, Pfizer announced that the European Medicines Agency had approved the companyメs 20-valent pneumococcal conjugate…

On Jan. 28, 2022, Merck and Ridgeback Biotherapeutics announced data from six preclinical studies demonstrating that molnupiravir, an…

On Jan. 18, 2022, Merck and Ridgeback Biotherapeutics announced the signing of a long-term supply agreement with the…

On Jan. 12, 2022, Pfizer announced positive top-line results from a Phase 3 study describing the safety and…

Our Heroes and Remembrance illustration has Maurice Ralph Hilleman and John Enders, pioneering developers of common vaccines, and…

On Dec. 24, 2021, Merck and Ridgeback Biotherapeutics announced that Japanメs Ministry of Health, Labor and Welfare had…

On Dec. 23, 2021, Merck and Ridgeback Biotherapeutics announced that the U.S. Food and Drug Administration (FDA) had…

On Dec. 23, 2021, Pfizer and BioNTech announced that they had submitted longer-term follow-up data from the companiesメ…

On Dec. 20, 2021, Pfizer and BioNTech announced an agreement had been reached with the European Commission (EC)…

On Dec. 8, 2021, Pfizer and BioNTech announced results from an initial laboratory study demonstrating that serum antibodies…

On Dec. 8, 2021, in a large-scale study of people from diverse ancestries, researchers narrowed down the number…
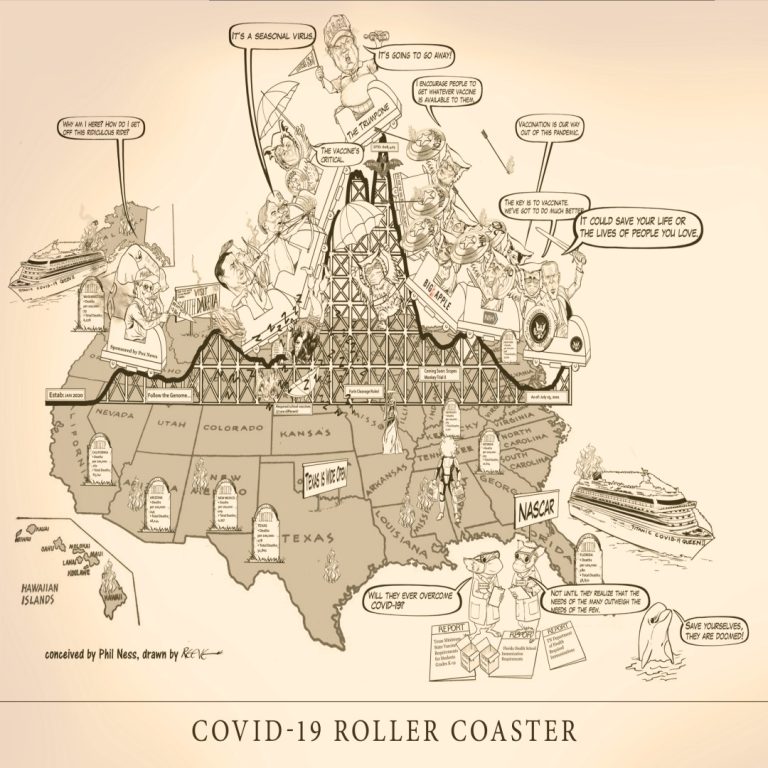
The COVID-19 Roller Coaster is a wild ride, strap yourself in and hold on… Cast of Characters: Senator…

On Nov. 5, 2021, Pfizer announced it was investigational novel COVID-19 oral antiviral candidate, PAXLOVID, significantly reduced hospitalization…

On Nov. 5, 2021, Chugai Pharmaceutical, announced that it had obtained approval from the Ministry of Health, Labour…

On Oct. 28, 2021, Pfizer and BioNTech announced that the U.S. government had purchased 50 million more doses…

On Oct. 21, 2021, Pfizer and BioNTech announced topline results from a Phase 3 randomized, controlled trial evaluating…

On Oct. 18, 2021, Pfizer and BioNTech announced that the European Medicines Agency’s Committee for Human Medicinal Products…

On Oct. 15, 2021, Pfizer and BioNTech announced they had submitted data supporting the vaccination of children 5…

On Oct. 12, 2021, National Resilience announced a strategic collaboration with Children’s Hospital of Philadelphia (CHOP) to implement…

On Oct. 4, 2021, Pfizer and BioNTech announced that the Committee for Medicinal Products for Human Use (CHMP)…

On Oct. 1, 2021, Merck and Ridgeback Biotherapeutics announced that molnupiravir (MK-4482, EIDD-2801), an investigational oral antiviral medicine,…
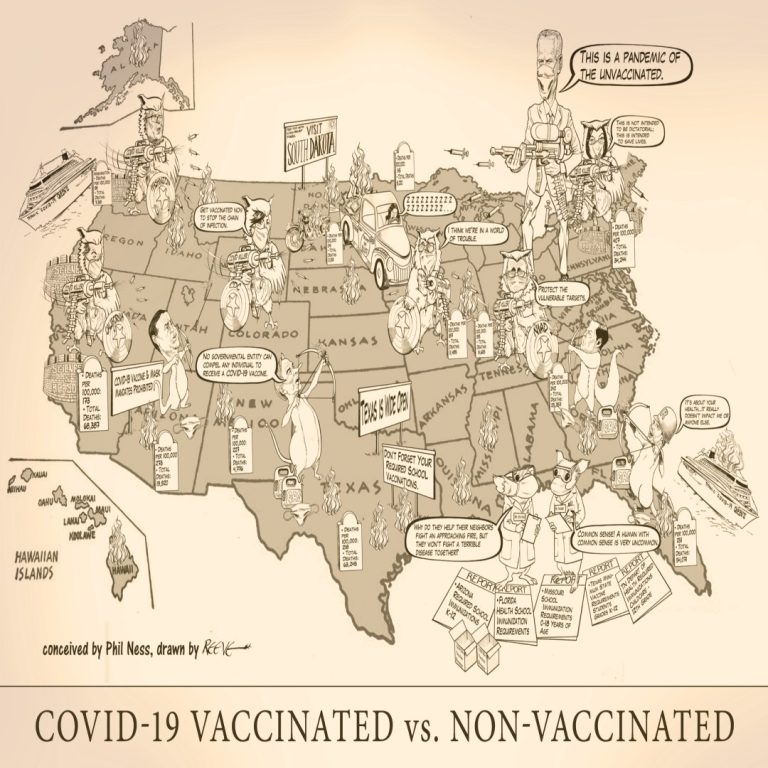
This cartoon illustrates the various issues facing individuals across the U.S. who are vaccinated against COVID-19 verus those…

On Sept. 29, 2021, Roche confirmed positive data from the phase II/III 2066 study, investigating Ronapreveル (casirivimab and…

On Sept. 29, 2021, Pfizer and BioNTech announced they had submitted data to the U.S. Food and Drug…

On Sept. 28, 2021, Sanofi announced that it had terminated plans for its own mRNA-based COVID-19 vaccine given…

On Sept. 14, 2021, Regeneron announced an agreement with the U.S. Department of Health and Human Services and…