US FDA approves AbbVie’s drug for a type of lung cancer
On May 14, 2025, The U.S. Food and Drug Administration (FDA) has approved AbbVie’s drug to treat adults…

On May 14, 2025, The U.S. Food and Drug Administration (FDA) has approved AbbVie’s drug to treat adults…

On May 9, 2025, Teal Health® announced the Food and Drug Administration’s (FDA) approval of the Teal Wand™,…

On May 7, 2025, researchers at the University College London (UCL) and King’s College London announced that an…
On May 6, 2025, in a first-of-its-kind surgery, a multidisciplinary team led by a University of Maryland Medical…

On May 5, 2025, The European Union (EU) and France announced half a billion euros worth of incentives…

On May 2, 2025, researchers at the Wellcome Sanger Institute, UCL, and the University of Cambridge announced they…

On May 1, 2025, the U.S. National Institutes of Health (NIH), the world’s largest funder of biomedical research,…

On Apr. 23, 2025, researchers from the Wellcome Sanger Institute, University of California San Diego and their collaborators…

On Apr. 21, 2025, the National Institutes of Health (NIH) reported that overall death rates from cancer declined steadily among…

On Apr. 21, 2025, LifeScienceHistory.com published the cartoon: “The National Institutes of Health (NIH) and U.S. Healthcare in…

On Apr. 11, 2025, the UK National Institute for Health and Care Excellence (NICE) recommended capivasertib (also called…
On Apr. 10, 2025, the U.S. Food and Drug Administration announced it is taking a groundbreaking step to…

On Apr. 8, 2025, the National Security Commission on Emerging Biotechnology delivered its major report and action plan…

On Apr. 3, 2025, a collaborative research led by investigators at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center…

On Apr. 1, 2025, layoff notices began arriving for thousands of employees of the sprawling U.S. Department of…

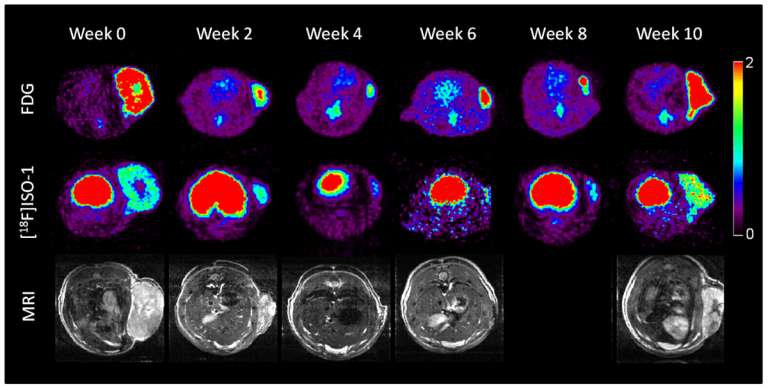
On Mar. 24, 2025, scientists at the UCLA Health Jonsson Comprehensive Cancer Center and the University of Toronto…

On Mar. 3, 2025, the Institute for Health Metrics and Evaluation reported that without urgent policy reform and…

On Feb. 24, 2025, breast cancer is the most common cancer in women worldwide, but survival chances vary…

On Feb. 19, 2025, the Cancer Prevention and Research Institute of Texas (CPRIT) announced that it had approved…

On Feb. 19, 2025, research from the Texas A&M College of Veterinary Medicine and Biomedical Sciences has discovered…

On Feb. 17, 2025, researchers from Texas Children’s Hospital in Houston announced that the four year old girl…

On Feb. 17, 2025, Stanford researchers have conducted the first large-scale screen of these inherited changes, called single…

On Feb. 5, 2025, scientists from the Cancer Dependency Map (DepMap) at the Broad Institute of MIT and…

Jan. 24, 2025, the U.S. Food and Drug Administration (FDA) pulled draft guidance from its website requiring companies…

On Jan. 15, 2025, the U.S. Food and Drug Administration (FDA) announced revoking the authorization for the use…

On Jan. 15, 2025, the U.S. Department of Commerce’s Bureau of Industry and Security (BIS) released an Interim…

On Jan. 13, 2025, scientists from the Francis Crick Institute, UCL, UCLH and Personalis announced they have found…

On Jan. 10, 2025, researchers at Roswell Park Comprehensive Cancer Center have identified a previously unknown cause of…
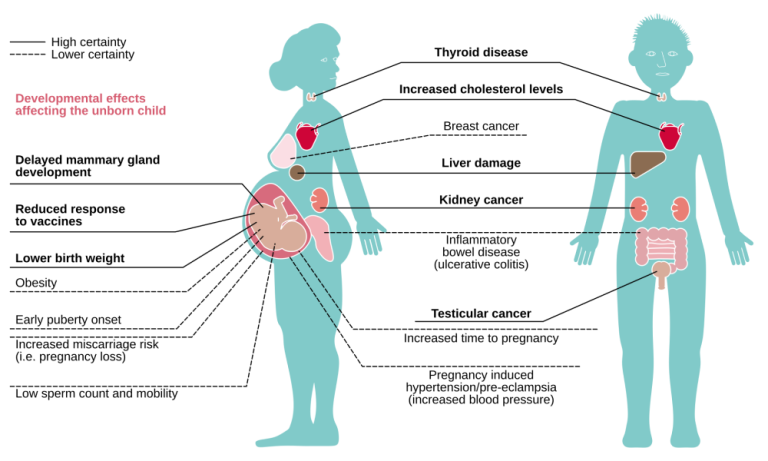
On Jan. 3, 2025, the U.S. Environmental Protection Agency announced the automatic addition of nine per- and polyfluoroalkyl…

On Jan. 3, 2025, the U.S. General Dr. Vivek Murthy released a new Surgeon General’s Advisory on Alcohol…