The largest-ever prostate cancer prevention study showed that Selenium and Vitamin E do not help prevent prostate cancer
On Dec. 9, 2008, in contrast with earlier research suggesting that dietary supplementation with selenium and vitamin E…

On Dec. 9, 2008, in contrast with earlier research suggesting that dietary supplementation with selenium and vitamin E…
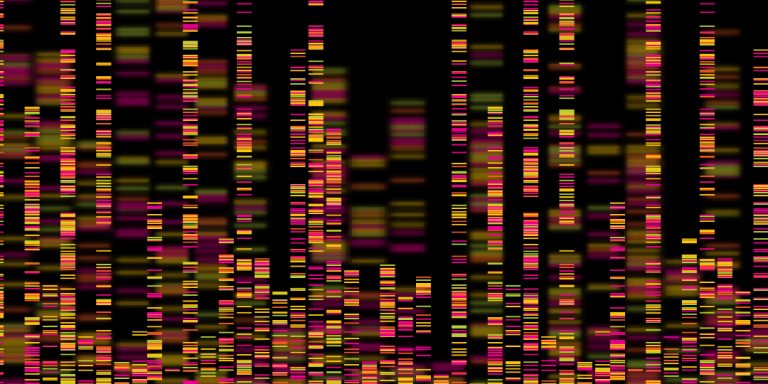
On Nov. 5, 2008, Siteman Cancer Center and The Genome Center researchers Timothy J. Ley, MD, Elaine Mardis,…
On Oct. 29, 2008, Oregon Health & Science University (OHSU) announced that Nike founder Philip H. Knight and…

On Oct. 8, 2008, the National Institutes of Health (NIH) announced it had awarded the University of Georgia…
On Sept. 14, 2008, the National Cancer Institute (NCI) researchers and colleagues have shown that increased expression of…

On Sept. 2008, a narrow region on chromosome 15 contains genetic variations strongly associated with familial lung cancer,…
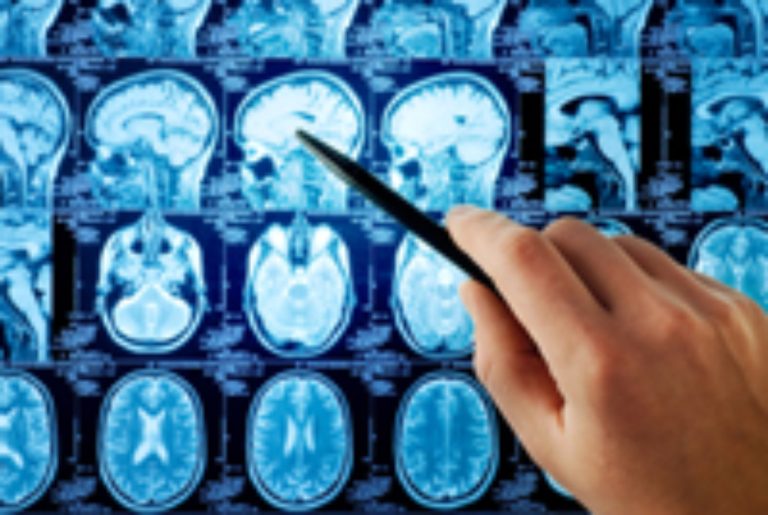
On Sept. 4, 2008, the Cancer Genome Atlas reported first results of comprehensive study of brain tumors. This…
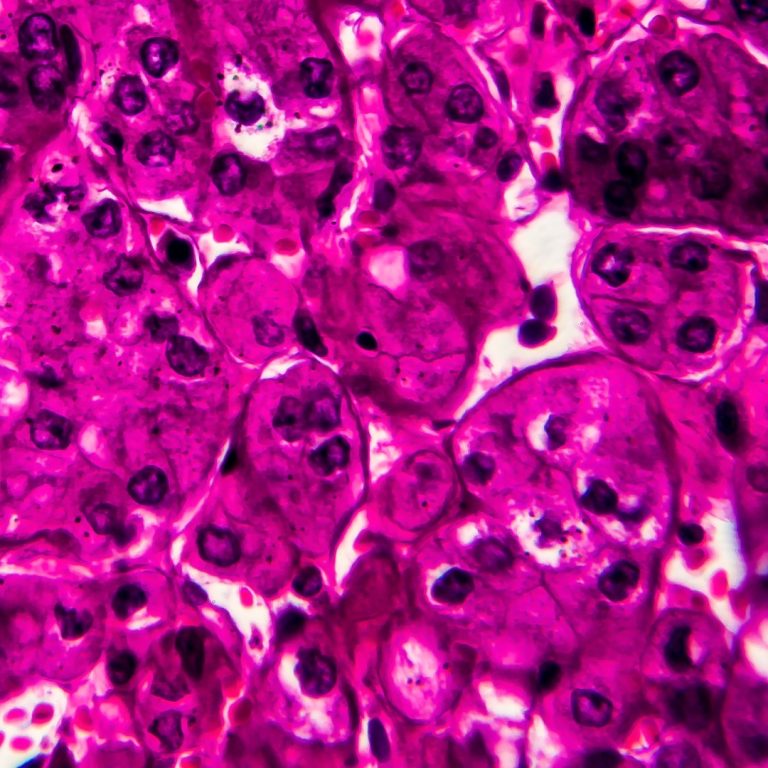
On Jul. 8, 2008, Stanford researchers announced discovered a molecule that kills kidney cancer cells. Ideally, the researchers…
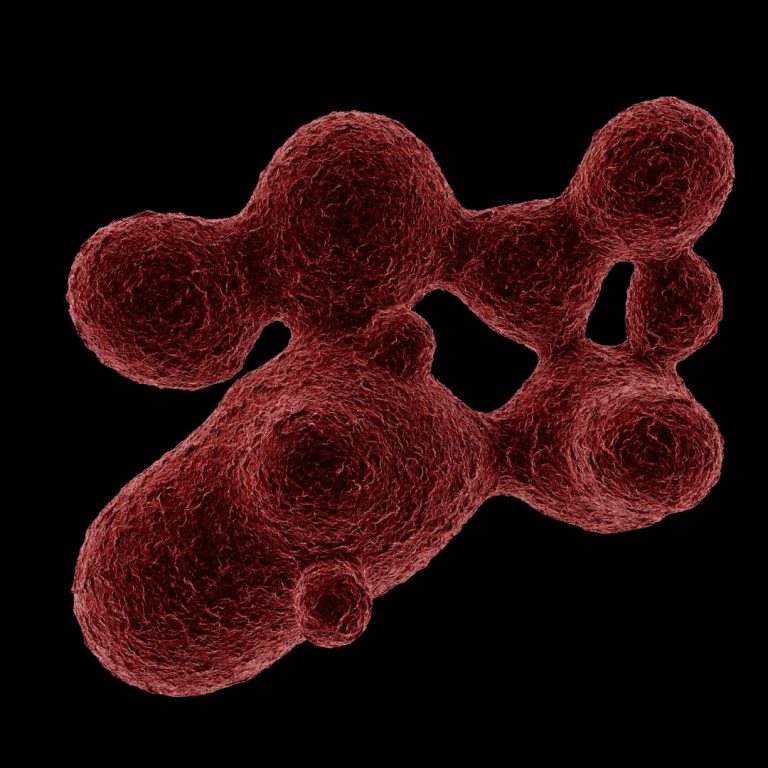
On Jun. 22, 2008, National Cancer Institute researchers announced that cells from a blood-borne cancer called multiple myeloma…

On Jun. 18, 2008, Fred Hutch researchers described the first successful use of a human patient’s cloned infection-fighting…

On May 13, 2008, the U.S. Congress passed the Breast Cancer and Environmental Research Act which amended the…

On Apr. 29, 2008, National Cancer Institute (NCI) researchers identified a pattern of gene activity in mice that…

On Mar. 6, 2008, DNA mutations found in a type of non-Hodgkin lymphoma that has a poor prognosis…

On Feb. 20, 2008, Lori and Jen-Hsun Huang of Los Altos Hills, California, announced a $5 million gift…
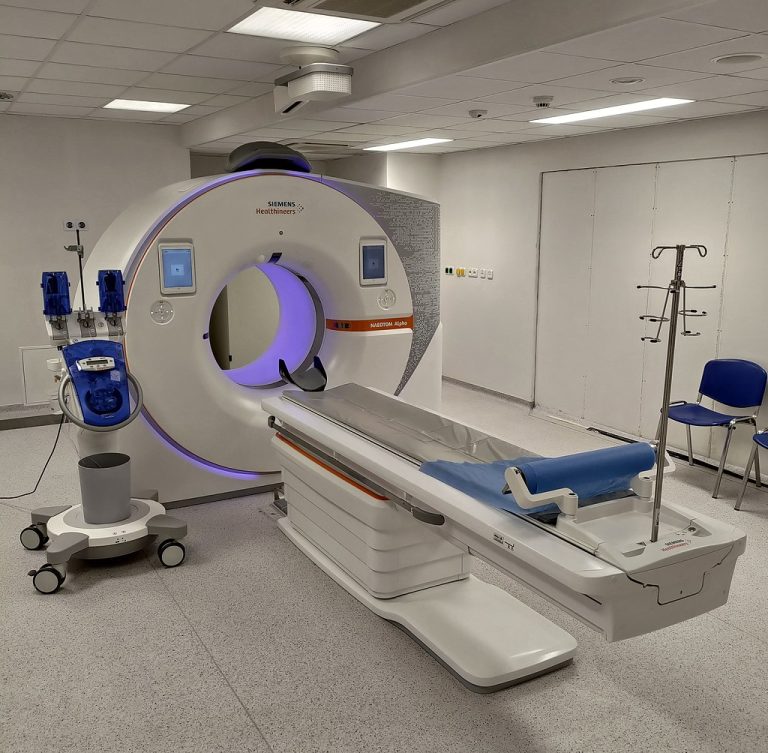
In 2008, results from a large multicenter study showed that the accuracy of computerized tomographic colonography (virtual colonoscopy)…

In 2008, the University of Maryland Marlene and Stewart Greenebaum Comprehensive Cancer Center (UMGCC) received NCI designation, a…
In 2008, the Tisch Cancer Institute (TCI) was founded through the generosity of James and Merryl Tisch. James…

In 2008, Evestra was spun-off as a private biopharmaceutical company from the Texas Biomed Department of Organic Chemistry….
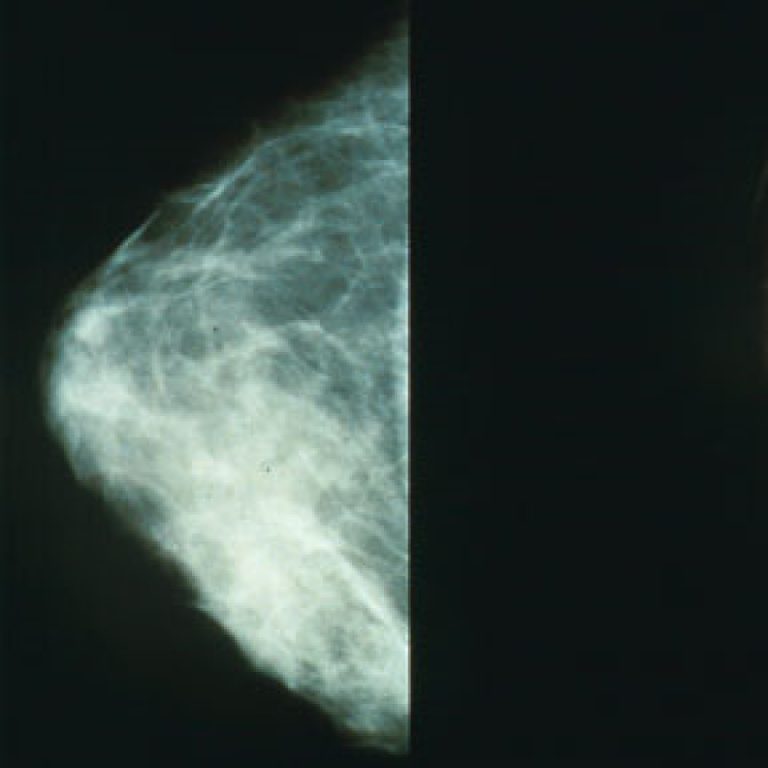
In 2008, the Seattle Cancer Care Alliance introduced the Mammovan, making digital mammography more accessible to women throughout…
On Dec. 12, 2007, the Breast Cancer Research Stamp Reauthorization Act extended through Dec. 31, 2011, provisions requiring…

On Dec. 6, 2007, the Cancer Therapy & Research Center became part of the UT Health Science Center…

On Nov. 27, 2007, a new National Cancer Institute (NCI) model for calculating invasive breast cancer risk, called…

On Nov. 19, 2007, the University of California at San Francisco Cancer (UCSF) Research Institute was renamed the…

On Oct. 30, 2007, the Barnes-Jewish Hospital (BJC) Institute of Health at Washington University was established, to house…
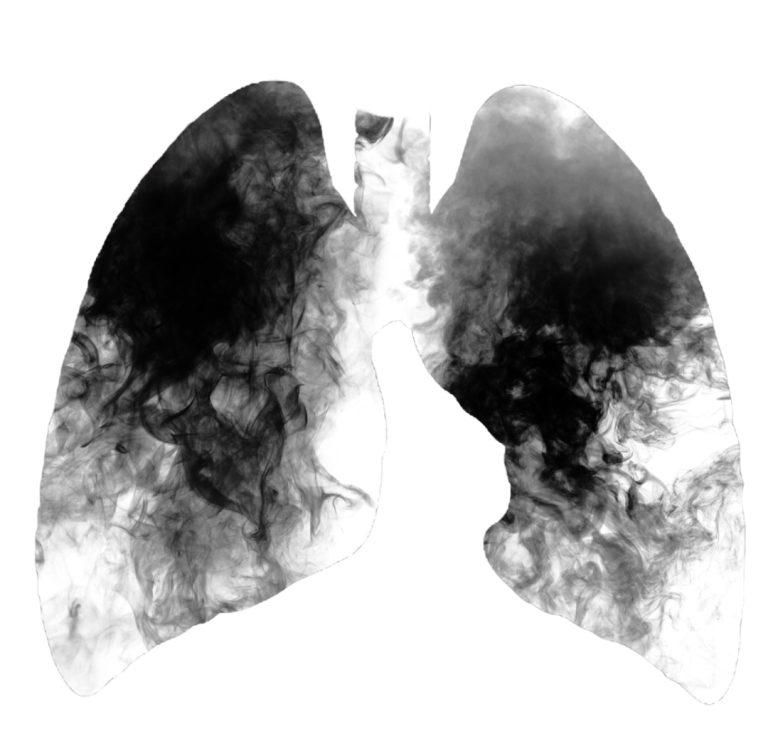
on Oct. 15, 2007, the annual report from the National Cancer Institute (NCI) showed that cancer death rates…

On Oct. 9, 2007, the Fred Hutchinson Business Alliance announced that the 2007 E. Donnall Thomas Medal of…

On Oct. 9, 2007, the David H. Koch Institute for Integrative Cancer Research at MIT was established with…

On Sept. 23, 2007, the Stanford Cancer Institute (SCI) was awarded “cancer center” designation from the National Cancer…

On Sept. 14, 2007, the U.S. Food and Drug Administration (FDA) announced it had approved Eli Lilly’s osteoporosis…

On Sept. 10, 2007, the National Cancer Institute (NCI) awarded a five-year, $12.5 million Specialized Program of Research…