Bezos family donated $35 million to Fred Hutch
On Mar. 30, 2017, the Fred Hutchinson Cancer Research Center announced that the Bezos family had donated $35…
On Mar. 30, 2017, the Fred Hutchinson Cancer Research Center announced that the Bezos family had donated $35…
On Dec. 3, 2016, Celgene, Dana-Farber Cancer Institute and the University of Arkansas for Medical Sciences announced the…

On Oct. 27, 2016, Roswell Park Cancer Institute announced it had received authorization from the U.S. Food and…
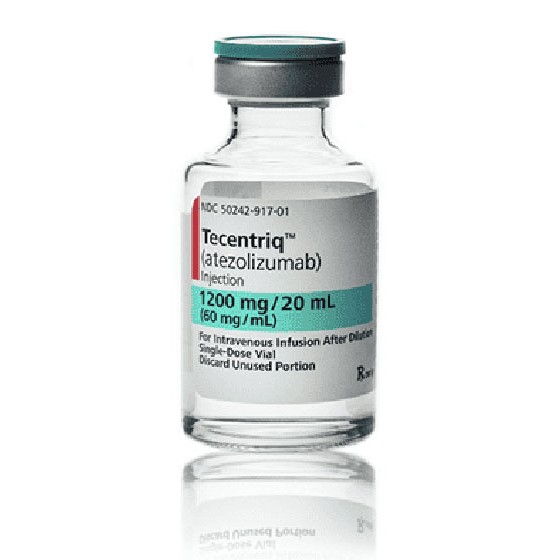
On Oct. 18, 2016, the U.S. Food and Drug Administration (FDA) approved TECENTRIQ® (atezolizumab) for the treatment of…

On Oct. 18, 2016, the University of Oregon (UO) announced the $1 billion Phil and Penny Knight Campus…

On Sept. 29, 2016, University of Southern California (USC) Trustee Marc Benioff ’86 and his wife, Lynne, gifted…

On Sept. 22, 2016, Vanderbilt University Medical Center became the world’s first site to treat a prostate cancer…

On Aug. 29, 2016, Scripps Health and MD Anderson Cancer Center announced a partnership agreement to create Scripps…
On Aug. 15, 2016, Siteman Cancer Center received a $10.4 million five-year grant to Washington University researchers and…

On Jul. 17, 2006, the SERM drug raloxifene (Evista) was found to reduce breast cancer risk for postmenopausal…

On Jul. 5, 2016, The Stanford Cancer Institute announced it had been designated a Comprehensive Cancer Center by…

On Jun. 16, 2016, The University of Michigan Board of Regents announced a $17.5 million commitment for cancer…

On May 19, 2016, President Barack Obama presented Mary-Claire King, Ph.D., a University of Washington professor of medicine…

On May 12, 2016, technology entrepreneur and founder of Oracle, Larry Ellison announced the donation $200 million to…
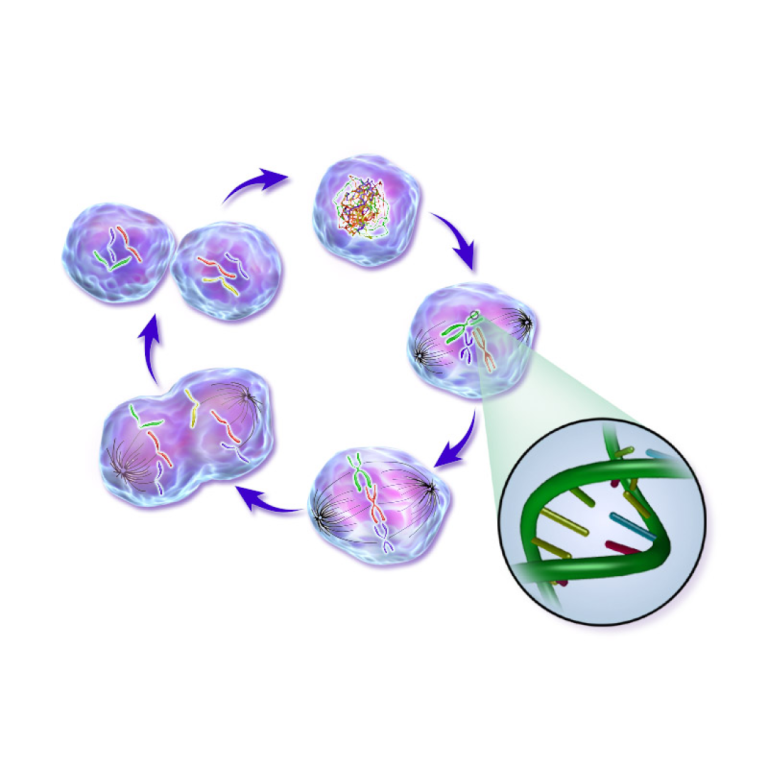
On Apr. 18, 2016, a team of Oklahoma Medical Research Foundation (OMRF) researchers led by Gary Gorbsky found…

On Mar. 3, 2016, the University of Oregon (UO) announced receipt of a $10 million gift from Tim…
On Feb. 17, 2016, MD Anderson Cancer Center was awarded $14 million from the Cancer Prevention and Research…

On Feb. 17, 2016, the Cancer Prevention and Research Institute of Texas (CPRIT) awarded grants totaling $26,000,000 to…

On Jan. 12, 2016, a consortium of industry, government, and academia announced the launch of the Cancer MoonShot…

On Jan. 11, 2016, the U.S. Preventive Services Task Force (USPSTF) recommended biennial screening mammography for women aged…
On Apr. 11, 2016, the U.S. Food and Drug Administration (FDA) announced that it had granted accelerated approval…

In 2016, Siteman and St. Louis Children’s Hospital established Siteman Kids at St. Louis Children’s Hospital, focused on…
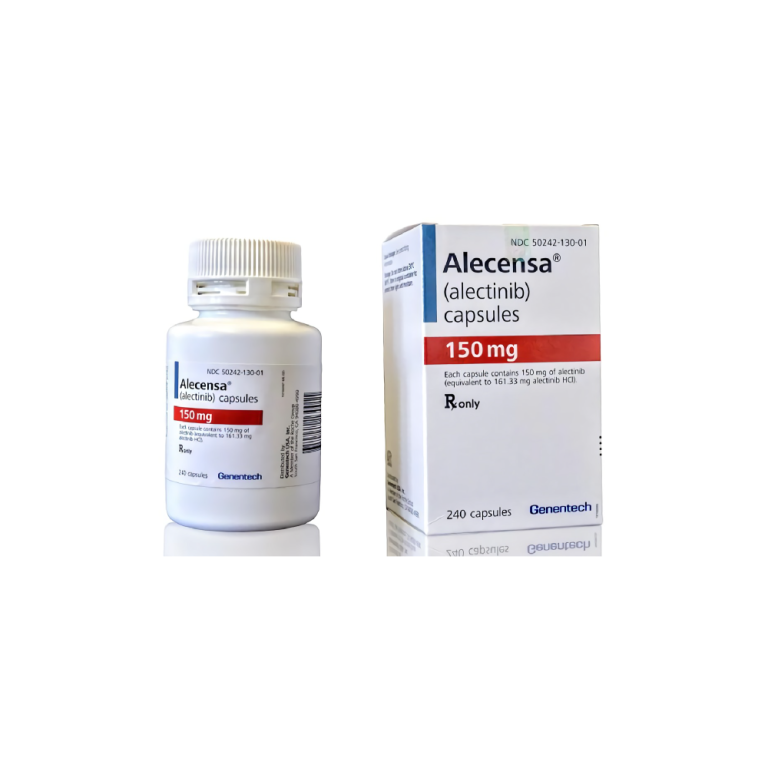
On Dec. 11, 2015, Genentech announced that it’s drug Alecensa (alectinib) was approved by the U.S. Food and…

On Nov. 18, 2015, the estate of Glenn Korff gifted $5 million to the Fred & Pamela Buffett…

On Nov. 5, 2015, TapImmune, (TPIV) announced it had relocated its corporate headquarters from Seattle, Washington, to Jacksonville,…

On Oct. 21, 2015, the Omar Boraie Chair in Genomic Science was established at Rutgers Cancer Institute. The…

On Oct. 1, 2015, the National Cancer Institute (NCI) awarded a $2.8 million grant five-year grant to researchers…

On Aug. 12. 2015, the University of New Mexico Cancer Center received National Cancer Institute (NCI) designation. Cancer…

On Jul. 30, 2015, Baylor Cancer Center received comprehensive designation from the National Cancer Institute (NCI). The Center…

On Jul. 10, 2015, the National Cancer Institute (NCI) announced it had awarded the UT Southwestern Medical Center’s…