NIH software assembled complete genome sequences on-demand
On Feb. 16, 2023, National Institutes of Health (NIH) scientists announced they had developed and released an innovative…

On Feb. 16, 2023, National Institutes of Health (NIH) scientists announced they had developed and released an innovative…
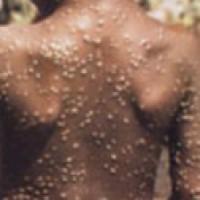
On Feb. 14, 2023, scientists from the National Institute of Allergy and Infectious Diseases announced they had developed…

On Feb. 10, 2023, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for…

On Feb. 9, 2023, the National Institutes of Health (NIH) announced that a new large-scale genetic analysis found…

On Feb. 7, 2023, an NIH funded study by University of North Carolina reported that Bivalent booster vaccines…
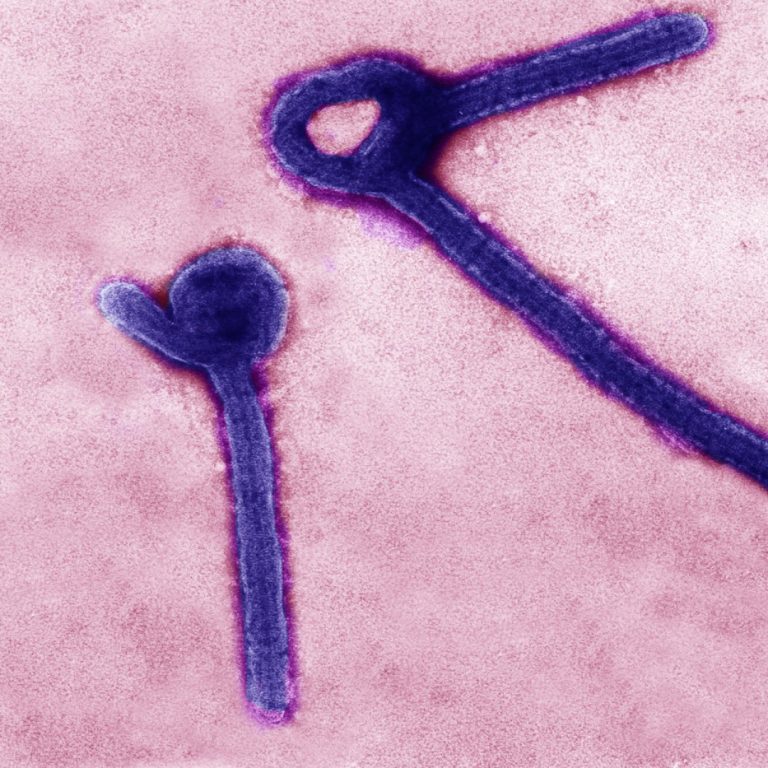
On Feb. 2, 2023, a National Institutes of Health research group with extensive experience studying ebolavirus countermeasures successfully…

On Feb. 1, 2023, researchers funded by the National Institutes of Health announded they had developed a new…
On Jan. 25, 2023, it was reported that adults living in rural areas of the United States have…

On Jan. 18, 2023, the National Institutes of Health (NIH) announced that an investigational HIV vaccine regimen tested…
On Dec. 5, 2022, the Fred Hutchinson Cancer Center announced that according to a new meta-analysis women were…

On Nov. 16, 2022, researchers at the National Institutes of Health (NIH) announced they had successfully identified differences…
On Nov. 7, 2022, the NHS Blood and Transplant (NHSBT) reported that red blood cells grown in a…
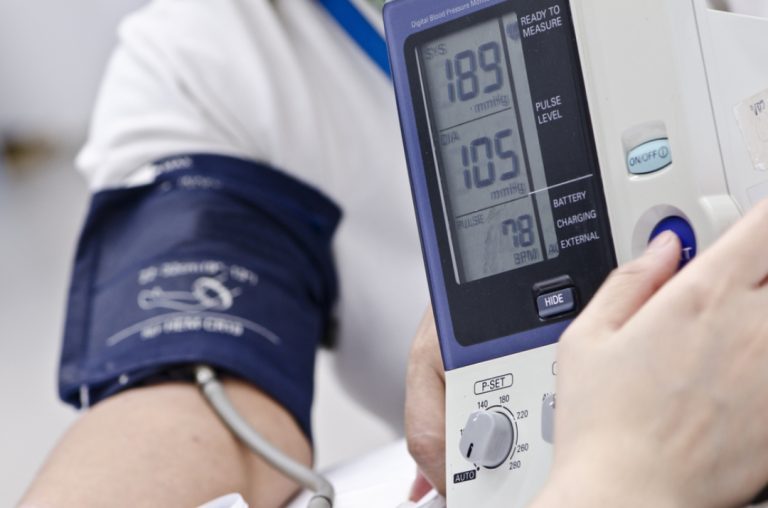
On Nov. 1, 2022, the National Institutes of Health (NIH) announced that adults with hypertension saw a small,…

On Oct. 27, 2022, the National Institutes of Health (NIH) announced that overall cancer death rates continued to…

On Oct. 26, 2022, the NIH announced it had awarded the University of Southern California (USC) $11.7 million…

On Oct. 25, 2022, the National Institutes of Health (NIH) announced that the University of Missouri had been…
On Oct. 24, 2022, the National Institutes of Health announced that starting antiretroviral treatment (ART) early in the…
On Oct. 20, 2022, the National Institutes of Health announced that a three-dose course of the hepatitis B…

On Sept. 27, 2022, the National Institutes of Health (NIH) announced that a large international study had confirmed…
On Sept. 27, 2022, the National Institutes of Health (NIH) announced it had launched a program to better…

On Supt. 22, 2022, with a five-year, $126 million grant from the National Institutes of Health (NIH), a…

On Sept. 15, 2022, the National Institutes of Health announced that a research team funded by the NIH…
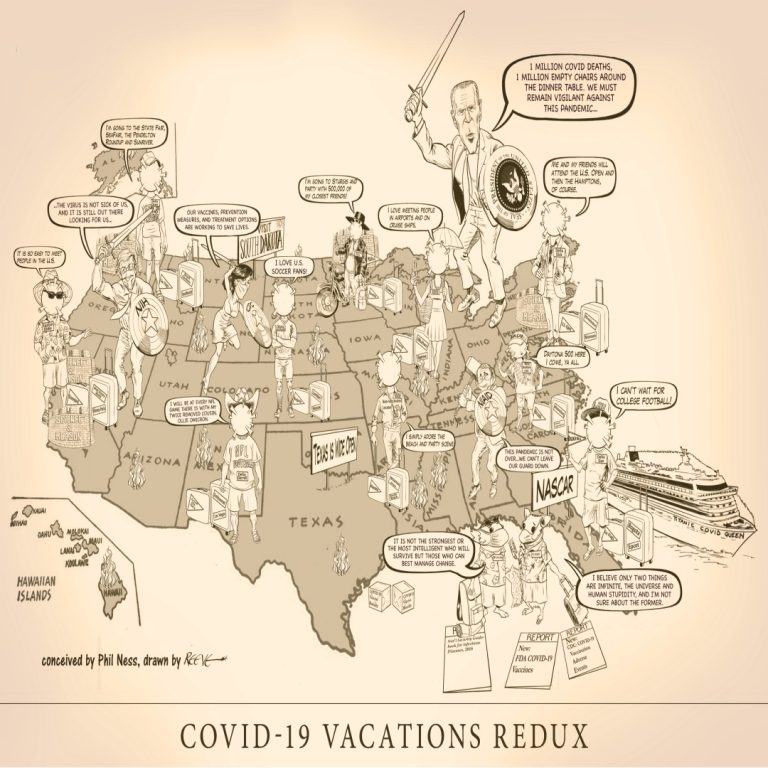
COVID-19 Vacations Redux iIlustrates once again the pits and perils vacation travelers face as they begin their Summer…
On Jul. 19, 2022, the NIH announced that although COVID-19 booster vaccinations in adults elicit high levels of…

On Jul. 11, 2022, Novavax announced an agreement with the U.S. Department of Health and Human Services (HHS),…
On Jul. 11, 2022, the National Institute of Allergy and Infectious Diseases (NIAID) launched an early-stage clinical trial…
On Jul. 5, 2022, the National Institutes of Health and National Cancer Institute announced COVID-19 was the third…
On Jun. 28, 2022, the National Institutes of Health (NIH) announced that a Phase 1 clinical trial of…

On Jun. 2, 2022, the National Institutes of Health (NIH) reported that the Age-Related Eye Disease Studies (AREDS…

On Jun. 1, 2022, the U.S. National Institutes of Health announced that an NIH funded study had found…