W. Kimryn Rathmell, M.D., Ph.D., began work as 17th director of the National Cancer Institute
On Dec. 18, 2023, W. Kimryn Rathmell, M.D., Ph.D., began work as the 17th director of the National…

On Dec. 18, 2023, W. Kimryn Rathmell, M.D., Ph.D., began work as the 17th director of the National…

On Dec. 13, 2023, National Institute of Allergy and Infectious Diseases (NIAID) scientists announced findings published in the…
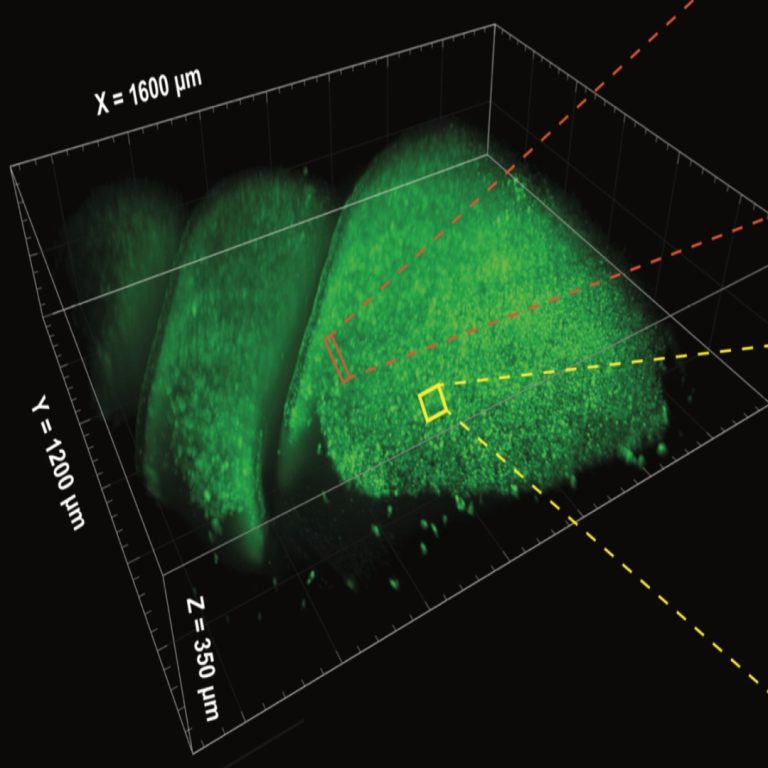
On Dec. 13, 2023, for the first time ever, an international team of researchers announced they had created…

On Nov. 22, 2023, the U.S. Food and Drug Administration (FDA) issued the final guidance COVID-19: Developing Drugs…
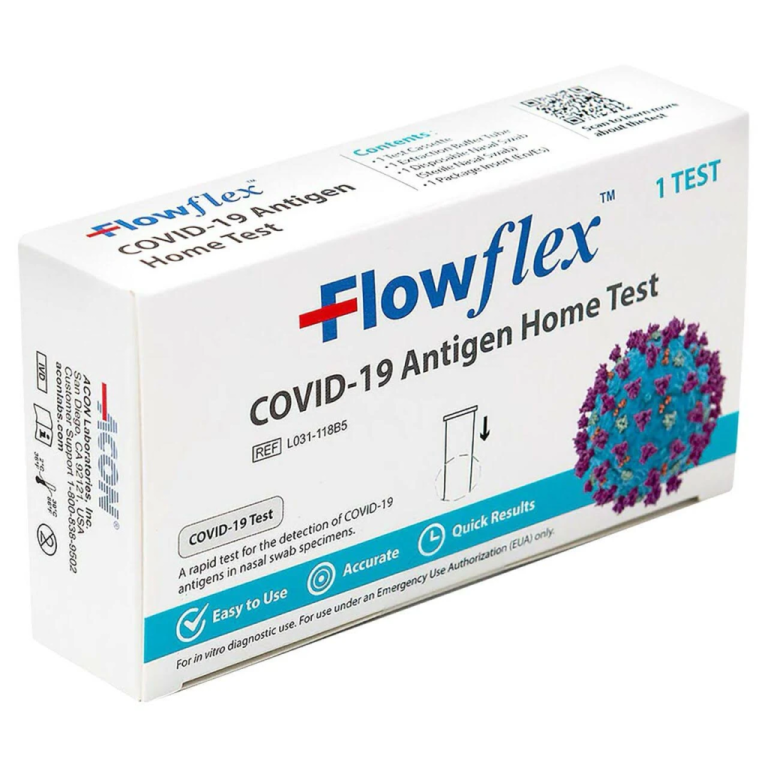
On Nov. 9, 2023, the U.S. Food and Drug Administration (FDA) cleared for marketing the first over-the-counter (OTC)…

On Nov. 9, 2023, the National Institute of health (NIH) announced that Monica M. Bertagnolli, M.D., had begun…

On Oct. 13, 2023, the National Institute of health (NIH) announced that an investigational drug, encaleret, restored calcium…

On Oct. 12, 2023, the National Institute of health (NIH) announced that a group of international scientists had…

On Sept. 29, 2023, through the Future Manufacturing program, the U.S. National Science Foundation (NSF) announced targeted investments…

On Sept. 26, 2023, the Advanced Research Projects Agency for Health (ARPA-H), an agency within the U.S. Department…
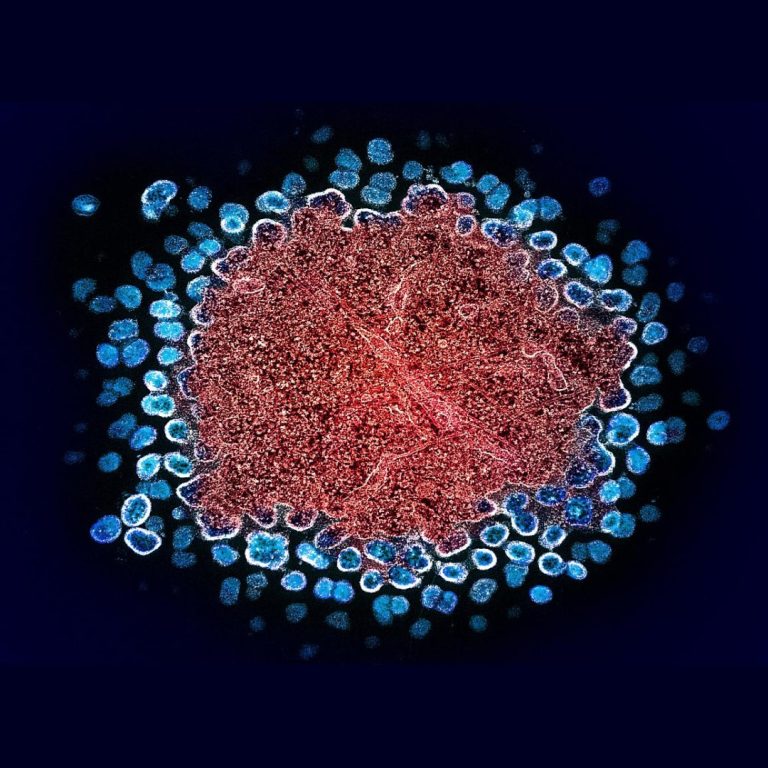
On Sept. 20, 2023, the National Institutes of Health announced that a trial of a preventive HIV vaccine…

On Sept. 12, 2023, the National Institutes of Health announced it was establishing the Multi-Omics for Health and…

On Sept. 11, 2023, researchers at the National Institutes of Health (NIH) reported that living in an area…
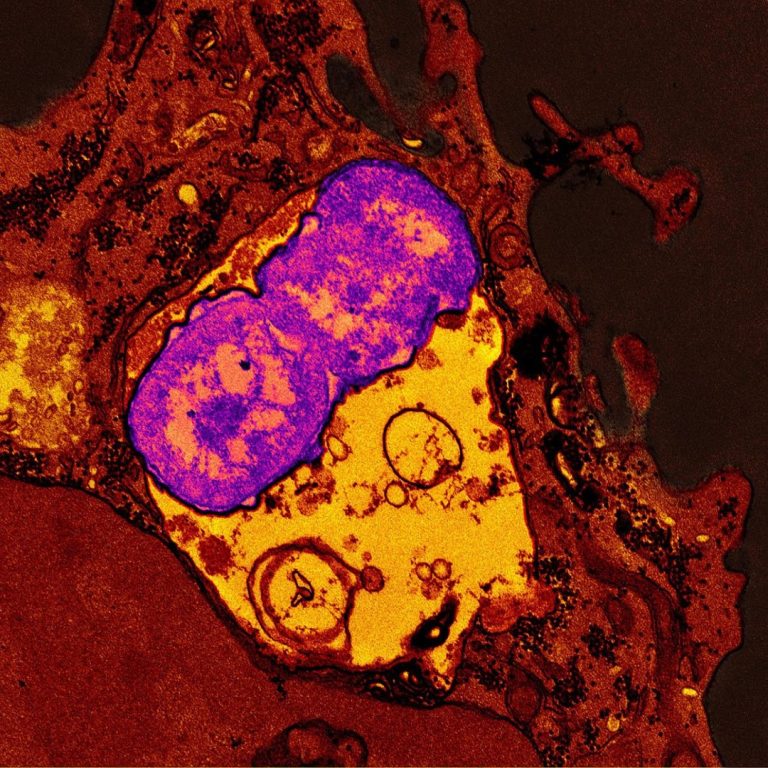
On Sept. 6, 2023, the National Institutes of Health (NIH) announced it was investgating ‘hypervirulent’ strains of the…
On Aug. 17, 2023, the National Institutes of Health announced it had awarded $24 million in first-year funding…

On Aug. 7, 2023, a National Institute of Health supported study found that in 2021, an estimated 2.5…

On Jul. 31, 2023, the U.S. Department of Health and Human Services announced the formation of the Office…

On Jul. 19, 2023, the National Institute of Health announced that a nationwide research team had created the…
On May 8, 2023, scientists at the National Institutes of Health (NIH) announced that they had identified new…
On May 5, 2023, the National Institutes of Health reported that a study had identified features of Long…
On May 3, 2023, National Institutes of Health (NIH) scientists announced that they had uncovered new details on…
On Apr. 27, 2023, a NIAID-led Phase 2 trial compared two Pfizer bivalent mRNA COVID-19 boosters found that…
On Apr. 11, 2023, a National Institutes of Health (NIH) clinical trial was stopped early because a daily…

On Apr. 4, 2023, National Institutes of Health (NIH) funded research was announced from the University of Rochester…

On Apr. 3, 2023, the U.S. Department of Health and Human Services (HHS) released a National Cancer Plan,…

On Mar. 21, 2023, the Centers for Disease Control and Prevention announced the acquisition of a site in…

On Mar. 20, 2023, NIH scientists announced that the magnitude and quality of a key immune cellメs response…

On Mar. 14, 2023, NIH researchers announced they had developed a new imaging tool, called electromyometrial imaging (EMMI),…

On Feb. 28, 2023, a team from the University of Sydney reported that a flu virus found in…

On Feb. 24, 2023, Moderna announced will make certain contingent development, commercial and regulatory milestone payments to the…